In 2022, the UK’s Cell and Gene Therapy Catapult (aka CGT Catapult) celebrated its tenth anniversary (congratulations!) marking 10 years of accelerating innovation in cell and gene therapies (CGTs) in the UK. Much like CCRM, CGT Catapult was established as an ecosystem-building organization. CGT Catapult was founded with the mission to grow a viable and sustainable cell therapy industry in the UK by enabling access to finance and providing clinical and technical expertise to enable the translation of cell therapies. (If you’d like to read a comparison between CGT Catapult, CCRM and the California Institute for Regenerative Medicine, see this post by Stacey Johnson.)
In March of 2022, CGT Catapult worked closely with the UK’s CGT industry to publish the National Cell and Gene Therapy Vision for the UK, outlining recommendations to ensure the UK’s ecosystem maintains its global CGT advantage. The report examines how the UK can scale up its manufacturing capacity; how therapeutics developers can be reimbursed; how adoption and best practice can be promoted within the UK’s National Health Service; and how Health Technology Assessment bodies can best process advanced therapies.
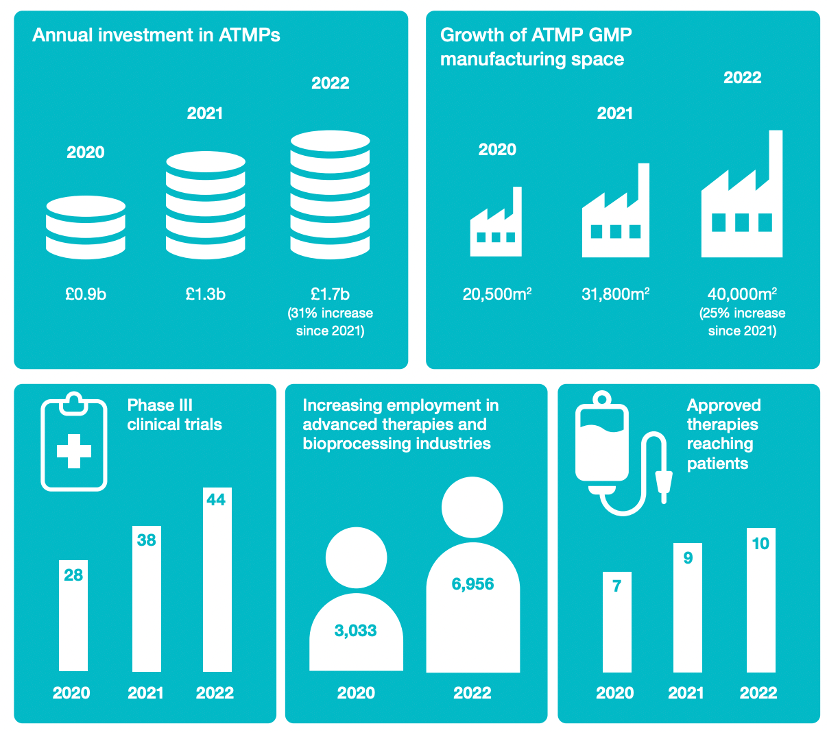
In November 2022, CGT Catapult published its Annual Review, reinforcing this vision of a thriving industry delivering life changing advanced therapies to the world. The report highlights how investment in advanced therapy medicinal products (ATMPs) and manufacturing space for the production of these ATMPs have both almost doubled since 2020. These developments come at an opportune time, with therapies increasingly reaching the Phase III clinical trial stage or graduating to regulatory approval and reaching patients via commercialization.
UK advanced therapy industry growth and CGT Catapult’s impact: CGT Catapult 2022 Annual Review
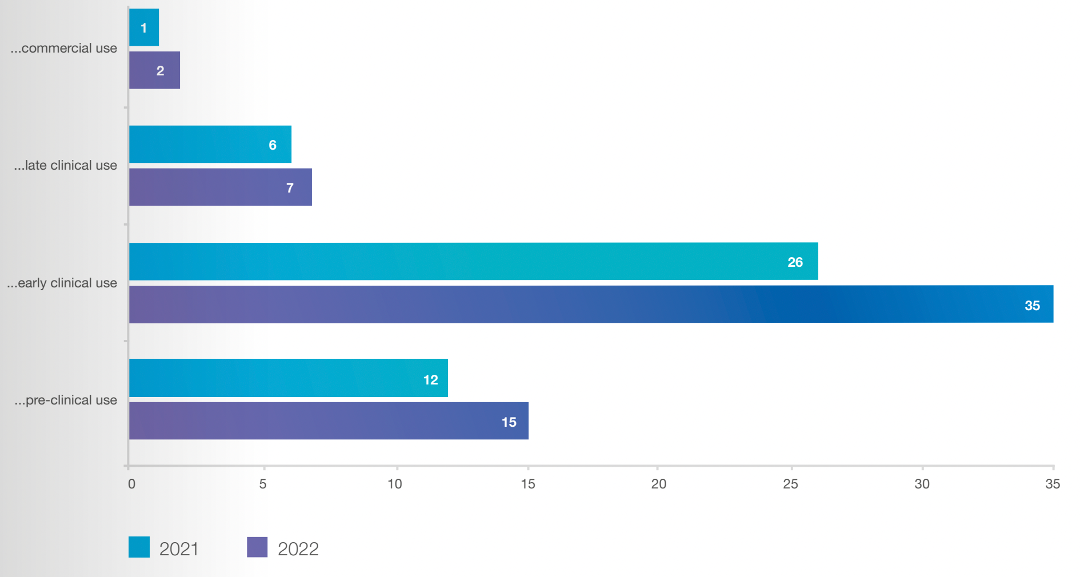
Looking at the UK’s national manufacturing picture, CGT Catapult’s Cell and Gene Therapy GMP Manufacturing in the UK report outlines the growth of the UK’s CGT manufacturing capacity, which has increased by 14 per cent since their 2021 report. The 2022 report notes that a site with a utilization of 80-90 per cent is generally considered to be full (and thus unable to take on significant new projects), and that average national utilization (booked capacity) in the UK is currently standing at 67 per cent – good news for UK therapeutics developers who are ready to initiate a Good Manufacturing Practices (GMP) project.
The majority of therapies being manufactured in the UK are bound for early-stage clinical trials – not a surprise – and marketed therapies destined for commercial use account for the smallest amount. This proportion is likely to change in the coming years, with an increasing number of advanced therapies reaching late-stage clinical trials or receiving regulatory approvals and going into production for commercial use.
CGT Catapult’s Cell and Gene Therapy GMP Manufacturing in the UK report
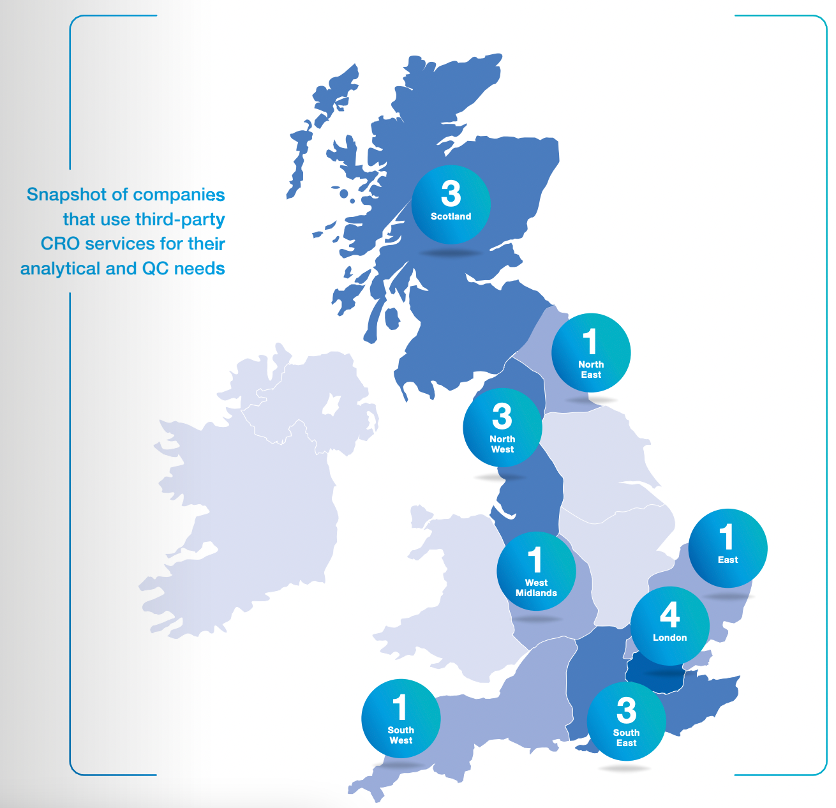
For the first time in 2021, CGT catapult asked therapeutics developers if they used a contract research organization (CRO) for their analytical and/or Quality Control needs. Of those that responded (n = 10) 60 per cent did use a CRO. In 2022, this figure is up to 74 per cent of companies that responded (see 2022 snapshot below).
CGT Catapult’s Cell and Gene Therapy GMP Manufacturing in the UK report
The story of industry growth seems to be reflected across most measured areas of Catapult’s 2022 reports. Notably, there has been significant growth in workforce figures, which reflects progress in strengthening talent pipelines with apprenticeship schemes and training programs. The number of apprentices in 2022 grew 46 per cent to 101. Total full-time employees of CGT manufacturing facilities in the UK grew 22 per cent, reaching a total of 1,696 employees.
CGT Catapult’s Cell and Gene Therapy GMP Manufacturing in the UK report
Capping the year, and aside from progress captured in CGT Catapult’s reports, on December 23, 2022, UCL was named a key strategic partner in the European Union’s new landmark €30M Centre for the Technologies of Gene and Cell Therapy (CTGCT). The CTGCT is scheduled to start in the second half of 2023, will run for six years, and be built in a new facility on the grounds of the National Institute of Chemistry in Ljubljana, Slovenia. Scientists at UCL will play key roles by helping to develop advanced drugs for the treatment of rare and incurable diseases, and act as a trusted experienced partner to the Centre to support the transfer of knowledge of ATMPs through to the clinical and commercial setting. Other partner institutions of the project include Utrecht University Medical Centre, Charité University Hospital Berlin, and Technical University Dresden.
“UCL are world-leading in the translation of these therapies and we are looking forward to sharing knowledge across the partnership in support of the next generation of scientists in Slovenia and across Europe,” said Dr. Jane Kinghorn in a UCL press release.
The team of eight leading experts at UCL, whose expertise will be leveraged as part of the project, includes Professor Qasim Rafiq, who was recently interviewed for the podcast Commercializing Living Therapies with CCRM, which examined how to train and retain tomorrow’s CGT workforce. Dr. Rafiq directs UCL’s new MSc degree program: Manufacture and Commercialisation of Stem Cell and Gene Therapies.
In addition to new collaborations forming, several new CGT manufacturing facilities are set to come online in the UK in 2023-24. The CGT Catapult’s Edinburgh facility will add 3,500 sq ft of high-spec, non-GMP lab space to the UK cluster and is due to begin wet work in the second quarter of 2023. It will consist of two manufacturing labs and one analytical development lab, and will be located in the Edinburgh BioQuarter. The Edinburgh facility will build ATMP translational expertise in Scotland and the North of England. CGT Catapult also expects licensing of an initial ~ 1,770 sq ft multifunctional CGT facility in Braintree, Essex in 2023, followed by a ~2,800 sq ft expansion in 2024.
Other key facilities highlighted in the Cell and Gene Therapy GMP Manufacturing in the UK report that will bring additional manufacturing capacity online in 2023-4 include:
- Autolus’ multifunctional ~50,000 sq ft manufacturing headquarters, anticipated to come online in 2023;
- Adaptimmune’s new Oxford-based facility in 2023. This will be dedicated to the manufacture of iPSC-derived, genetically-edited allogeneic T cells for Adaptimmune’s clinical assets, and in partnership with products for Genentech and Astellas;
- eXmoor Pharma Concepts Ltd. plans to license its new state-of-the-art, ~65,000 sq ft multifunctional facility in Bristol, in 2024. The facility will be a dedicated CGT process development and clinical manufacturing facility;
- By 2023, NHS Blood and Transplant (NHSBT) Clinical Biotechnology Centre plans to add the manufacture of nucleic acids, lentiviral vectors and recombinant adeno associated viral vectors to its services. NHSBT Barnsley also plans to gain manufacturing authorization in 2023.
It’s encouraging to see how the rapid growth of Canada’s CGT ecosystem is mirrored by rapid growth and progress in the UK, with work ongoing to address issues including manufacturing capacity, talent and regulation, and innovative solutions in the works on both sides of the Atlantic.
Check in with us next week when we’ll be looking south of the border to recent developments in the American CGT ecosystem, with highlights from the Alliance for Regenerative Medicine, the California Institute for Regenerative Medicine, and the U.S. private sector.
Cal Strode
Latest posts by Cal Strode (see all)
- What’s in the mix for 2026: ARM’s State of the Industry briefing - January 28, 2026
- World AIDS Day: Update on HIV cure research and gene therapies - December 1, 2025
- Headwinds and tailwinds for cell and gene therapy under the second Trump administration - March 11, 2025







Comments