Let’s play a game. If you could choose, what would be your superpower? Invisibility is a popular one, as are super strength, the ability to read minds, and time travel (but we know that messing with time can be dangerous). This list of the best superpowers of all time ranks them in the context of helping a person win a battle and save lives. But what if we are choosing our superpower for everyday life?
My choice would be super memory (not on the list, by the way). I don’t need to have a “photographic memory” or the very rare condition Highly Superior Autobiographical Memory so that I can recall everything I’ve ever read or to visualize, in detail, a dinner I ate 30 years ago. I’d like to remember details of special events and important conversations, and be able to quote from books I’ve read or movies I’ve watched.
Memory is precious. Yes, it would overwhelm us to remember everything, and selective memory can protect us from past pain, but I am envious of people who have great memories. And, like many of us, I fear losing mine as I get older, although I do not suffer from athazagoraphobia.
World Alzheimer’s Day happens yearly on September 21. To recognize this, Toronto Life magazine featured members of the dream team at Krembil Brain Institute who are making exciting breakthroughs in neurodegenerative diseases, including Alzheimer’s Disease (AD).
These researchers are approaching the problem of AD from a variety of angles. Despite the fact AD was identified over a century ago (1906), and billions of dollars has gone towards research, we are no closer to a cure and current treatments only delay onset of the disease without actually stopping its progression.
The first Alzheimer drug, tacrine (Cognex (R)), was approved by the Food and Drug Administration (FDA) in 1993, but has since been discontinued. In the next decade, four additional drugs would be approved in the U.S. Canada has a total of four approved drugs – three are approved for mild to moderate AD and two treat the advanced form of the disease. (One treats all stages of the disease so is counted here twice.)
In June, 2021, the FDA granted approval to aducanumab (Aduhelm (TM)) using the accelerated approval pathway. According to the news release, aducanumab is the first therapy to target and affect the underlying disease process.
Harvard Health Publishing explains the treatment as follows: “Aducanumab is a monoclonal antibody engineered in a laboratory to stick to the amyloid molecule that forms plaques in the brains of people with Alzheimer’s. Most researchers believe that the plaques form first and damage brain cells, causing tau tangles to form inside them, killing the cells. Once aducanumab has stuck to the plaque, your body’s immune system will come in and remove the plaque, thinking it’s a foreign invader. The hope and expectation are that, once the plaques are removed, the brain cells will stop dying, and thinking, memory, function, and behavior will stop deteriorating.”
Before you get too excited, Biogen, the maker of aducanumab, is required to conduct a new randomized, controlled clinical trial to verify the drug’s clinical benefit. If the trial is unable to demonstrate this, the FDA may withdraw approval of the drug. Aducanumab is not yet approved for use in Canada. It is currently under review.
What progress is happening with cell and gene therapies, if any?
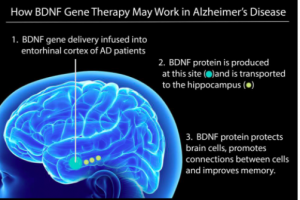
Image source: https://www.forbes.com/sites/carolineseydel/2021/03/12/clinical-trial-begins-for-gene-therapy-to-treat-alzheimers-disease/?sh=533017237171
Earlier this year, researchers at the University of California, San Diego launched a Phase I clinical trial to test the safety and efficacy of a gene therapy to deliver a key protein into the brains of people with AD to test whether it protects brain cells from dying.
According to UC San Diego Health, the protein is called brain-derived neurotrophic factor (BDNF). It is part of a family of growth factors found in the brain and central nervous system that supports the survival of existing neurons, and promotes growth and differentiation of new neurons and synapses. BDNF is particularly important in the entorhinal cortex and hippocampus – brain regions susceptible to degeneration in AD.
Because BDNF is a large molecule and difficult to get across the blood-brain barrier, the researchers will use a harmless adeno-associated virus (AAV2) that has been modified to carry the BDNF gene and inject it directly into the brain. Instead of having to take pills or get regular injections, your cells will be programmed to produce their own BDNF and this will keep AD away.
On the cell therapy front, there are a couple of updates to report.
In April 2021, Longeveron announced that it had favourable results from its Phase I clinical trial for Lomecel-B. The investigational bone marrow-derived medicinal signaling cell (MSC) showed an ability to slow cognitive decline in patients with mild AD. Patients also showed improvements in their ability to undertake activities of daily life. A Phase II trial is expected to begin before the end of 2021.
In 2019, Hope Biosciences began recruiting for a Phase I/II clinical trial using an autologous mesenchymal stem cell therapy (a therapy using your own cells), but recruitment was stopped due to the COVID-19 pandemic.
For progress in AD research, read what Signals blogger Lyla El-Fayomi shared in the August installment of Regenerative medicine news under the microscope.
Before you watch the following video, don’t forget to leave a comment about your favourite superpower!
In his TED Talk, University of Cambridge scientist Samuel Cohen, now CEO of Wren Therapeutics, says “Alzheimer’s is a disease, and we can cure it.” Learn why he thinks so by clicking on the video.
Stacey Johnson
Latest posts by Stacey Johnson (see all)
- Right Turn: Top 10 blogs from 2025 - January 9, 2026
- Right Turn: Season’s greetings and upcoming event - December 25, 2025
- Right Turn: Stem cell supplements: A growing market with growing risks - December 19, 2025






Comments