Cancer stem cell session panelists, clockwise from top left: Dr. Michael Rudnicki (moderator), and presenters Drs. Colin Hammond, Courtney Jones and Cédric Blanpain.
While I have attended several Till & McCulloch Meetings in the past as a trainee and presenter, I have now had the privilege of attending as a cancer stem cell enthusiast and blogger – and I’m excited to share my perspective on some of the amazing talks!
If you were unable to tune in for the opening virtual plenary session on cancer stem cells on Monday, November 15, we heard about research on cancer stem cell metabolism, the epithelial-mesenchymal transition, and cell cycle modulation. The session was moderated by Dr. Michael Rudnicki, and presentations were given by Drs. Courtney Jones, Cédric Blanpain, and Colin Hammond. Without further ado, on to the talks!
Targeting leukemia stem cell metabolism – Dr. Courtney Jones (University Health Network)
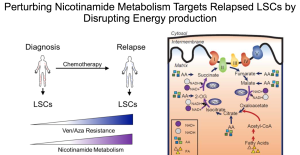
Focusing on acute myeloblastic leukemia (AML), Dr. Jones highlighted some exciting research that shows differences in the metabolism between leukemia stem cells (LSCs) derived from patients with newly diagnosed AML, and those with relapsed AML. This work was inspired by the observation that relapsed AML patients appear to be refractory to chemotherapies that target oxidative phosphorylation, the “typical” cellular metabolic process. Dr. Jones was curious as to which alternate metabolic pathway were relapse LSCs using, and could this pathway be exploited as a novel therapeutic target?
Her group found that nicotinamide (an important component in several metabolic pathways) was present in much higher levels in relapsed versus diagnosis LSCs. Functional relevance was demonstrated in two ways. First, when diagnosis LSCs were pretreated with nicotinamide, followed by a typical anti-oxidative phosphorylation chemotherapy, they became resistant to the chemotherapy, in a pattern similar to that of relapse LSCs. Second, when relapse LSCs were treated with a small molecule that inhibits nicotinamide metabolism, the cells lost their “stemness,” and became more like “regular” leukemia cells. They further showed that by inhibiting the preferred metabolic pathways of relapse LSCs, the cells became sensitive to standard oxidative phosphorylation chemotherapies once more.
These findings present some unique treatment options for relapsed AML patients. In fact, Dr. Jones ended her talk with the exciting announcement of a new Phase I clinical trial incorporating the use of their small molecules in the treatment of relapsed AML.
Mechanisms regulating tumour transition states – Dr. Cédric Blanpain (Université Libre de Bruxelles)
Dr. Blanpain’s research is based on the concept of intratumour heterogeneity: tumours are comprised of cells with variations in “appearance” and function. In particular, Dr. Blanpain is interested in those cell types that increase tumour malignancy and can lead to metastasis in skin squamous cell carcinoma.
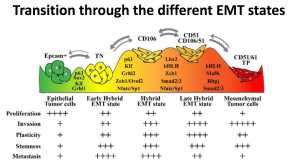
The mesenchymal (identifiable as Epcam-) cells of solid tumours are of particular interest, as they possess increased migratory and invasive properties, and are further thought to be the cells that can lead to tumour metastasis. While the majority of a solid tumour is comprised of the relatively “innocuous” epithelial cell (Epcam+), these cells can undergo an epithelial-mesenchymal transition (EMT), becoming the more malignant mesenchymal cell.
Dr. Blanpain has taken steps to demonstrate that several cell populations are present during this transition. Rather than a simple dichotomy of Epcam+ and Epcam-, three additional cell surface markers (CD51, CD61, and CD106) can be used to further stratify the various cell populations that appear during the EMT. Furthermore, each of these cell populations have variations in several functions that are required for tumour metastasis, invasion and growth.
His group is currently working on identifying and characterizing the genes responsible for regulating the transitions between the different cell types, in the hopes that this will identify future therapeutic targets that can prevent tumour metastasis. So far, they have found a few genes that play a major role in regulating the transitions between the different states. The next steps involve addressing the question of how these key genes regulate the transitions and, ultimately, could the genes be exploited to make the tumour vulnerable to new therapies?
Primitive human hematopoietic “CD49f” cells from normal donors display an aging-associated reduction in mitogen sensitivity and a mechanistically related elongation of their cell-cycle transit time – Dr. Colin Hammond (BC Cancer Research Centre)
The last talk explored how transitions through the phases of the cell cycle differed in hematopoietic stem cells (HSCs) throughout aging. Dr. Hammond works with a very rare HSC population (CD49f+ cells) and compares HSCs derived from normal cord blood samples and healthy adult donors.
Dr. Hammond demonstrated that adult HSCs seem to be “stuck” in the G1 phase of the cell cycle (the phase where the cell grows and makes mRNA and protein in preparation for DNA replication), when compared to their cord blood counterparts. This “delay” in transitioning into the next phase of the cell cycle seems to be related to a decreased sensitivity to growth factors, and reduced deactivation of checkpoint inhibitors; more simply, the adult HSCs require more “GO” signal than their cord blood counterparts. It makes sense that “young” and “adult” HSCs would have differences in their proliferative capacity: the body’s needs for cell growth are much higher in our infantile versus adult years.
Dr. Hammond hopes to further characterize the mechanisms that govern this slowing of cell cycle progression through aging, and plans to incorporate genomic studies comparing the different HSC populations. If we apply this line of thinking to leukemia stem cells (LSCs), perhaps this will allow for the discovery of new treatments that will slow/prevent the growth of LSCs by delaying their ability to progress through the cell cycle.
Commentary:
When I first read the agenda for the cancer stem cell session, I couldn’t imagine how leukemia stem cell metabolism, various epithelial-mesenchymal transition states, and cell cycle transit time in aging hematopoietic stem cells could relate to each other. But now after having listened to the talks, I couldn’t imagine how each of those concepts are not inextricably linked. It just seems so likely that different metabolic pathways are recruited as cells transition through their various states, and that in each of these states the cells undergo cell cycle transit changes to prepare them for their new roles and niches. Maybe we’ll see some new collaborations from these independent groups in the future!
Sara M. Nolte
Latest posts by Sara M. Nolte (see all)
- Ottawa researchers make tumour-marker negative cancers positive with oncolytic viruses - December 19, 2024
- Cell-based therapy approved as alternative to standard UCB transplant for hematological malignancies - June 21, 2023
- Finding the perfect match: breaking down the science of organ and stem cell transplant matching - April 18, 2023






Comments