In a field as fast-paced as regenerative medicine, it’s vital that policy isn’t always playing catch-up. I caught up with Amy Zarzeczny’s presentation at the 2021 Till & McCulloch Meetings to find out how policy can stay ahead — or at least keep pace with — emerging regenerative medicine technologies.
In her presentation “Regenerative medicine policy in Canada – looking to the future,” legal expert Amy Zarzeczny LLM, an Associate Professor at the University of Regina, shared what she considers four key pillars of strong future regenerative medicine policy. Keen to emphasize that these pillars are not intended as a comprehensive or exclusive list, Zarzeczny outlined how her proposed four key pillars build on much of the great work that has already been done by the Stem Cell Network and other collaborators in the field.
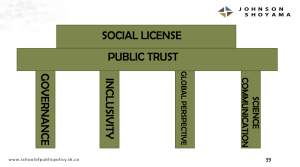
These pillars, which I will outline in brief, are governance, inclusivity, global perspective and science communication.
Governance
Using the definition “A framework of incentives, professional standards, regulations, norms and social expectations” as a starting point, Zarzeczny explored what this means in the context of regenerative medicine: “When we’re looking at regenerative medicine, I think the concepts of collaborative governance and distributive governance offer real value here because they give us ways to think about either deliberate or sometimes implicit ways that actors might co-ordinate their activities — or not.” Collaborative and distributive modes of governance stand out for their horizontal distribution of power between various actors, allowing policy to be produced in a way that values consensus over imposition. Zarzeczny emphasized the importance of considering the way in which power is distributed among different actors when contemplating what good governance looks like.
When considering clinical translation in regenerative medicine, key governance actors beyond government regulators include: the courts in their roles of interpreting legislation or assigning liability; scientific associations and their engagement in setting standards; health system leaders who make funding or uptake decisions around new technologies; patients and family advocacy organizations; and the media for the role they play in shaping public understanding and expectations of regenerative medicine.
Inclusivity
Outlining three critical priority areas – Truth and Reconciliation, Equity Diversity and Inclusion, and patient-oriented research – Zarzeczny emphasized that each are incredibly important, complex and highly nuanced. Underlining the importance of lived experience in informing future policy, Zarzeczny noted that “Nothing about me without me” is an increasingly central mantra that drives the work that’s done in these areas, and that ultimately more inclusive science will be stronger science, making these priorities a critical part of Canada’s future regenerative medicine policy.
“We have the considerations around what it will mean to implement the United Nations Declaration of the Rights of Indigenous People, how research communities can first acknowledge the historical racism and exclusion that we have seen take place as well as past research abuses, and what it will mean to move forward In a good way.”
Global perspective
A strong policy vision for Canada will require a global perspective because the challenges we face do not respect international borders and are sometimes even exacerbated by them. Citing the market for unproven stem cell interventions, Zarzeczny highlighted the importance of working together internationally on solutions.
“The market for unproven stem cell interventions stands as just one compelling example of a challenge that no one government, nation state or jurisdiction can deal with on its own. And as we see more regenerative medicine technologies progress to the clinic and develop interventions that stand to improve treatment options, we will also have some really important considerations of global health ethics and equity of access to consider.”
Effective science communication
Signals readers will need no convincing on this one; it’s something we covered last December, and, since the pandemic, it has been one of the hottest topics in health sciences. But we really need to look no further than stem cell research to see the power of misinformation, Zarzeczny points out, citing how quickly and easily legitimate enthusiasm about a promising field can blur into hype that risks fuelling public misunderstandings or unrealistic expectations around the state of the science. “We’ve seen that [hype] play into helping fuel access demands to stem cell interventions that have not progressed to clinically ready states, that are premature or simply unproven.”
A recent study by Leigh Turner found that more than four times as many businesses than were identified five years ago are selling stem cell products that are not FDA-approved and lack convincing evidence of safety and efficacy data, many of them advertising directly to patients via social media. “Social media and the power of online information has added incredible complexities to managing the information environment for individuals who are seeking information to make informed decisions about their own health, as well as for society more broadly,” Zarzeczny added.
Summarizing, Zarzeczny concluded that these four pillars could form a strong future regenerative medicine policy vision for Canada because they form the foundation of public trust in regenerative medicine and, ultimately, its social licence to operate and continue advancing. We’ve seen in the past how stem cell research can be hindered when the social license to operate is revoked, On August 9, 2001, then U.S. President George W. Bush introduced a ban on federal funding for research on newly created human embryonic stem cell lines. The ban remained in place until 2009 when President Barack Obama finally lifted it.
Cal Strode
Latest posts by Cal Strode (see all)
- World AIDS Day: Update on HIV cure research and gene therapies - December 1, 2025
- Headwinds and tailwinds for cell and gene therapy under the second Trump administration - March 11, 2025
- From innovation to international impact: ARM’s 2025 “State of the Industry Briefing” showcases maturing CGT industry - January 20, 2025






Comments