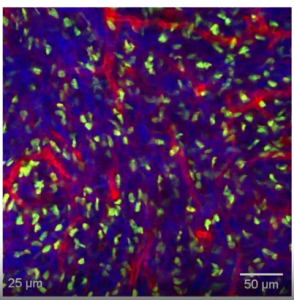
Fluorescently labelled cell types that form the mouse skin niche. (Screen capture from Prof. Valentina Greco’s talk during TMM2021.)
This year, the Till & McCulloch Meetings (TMM) featured two particularly exciting Plenary Sessions for those of us with a passion for the nitty-gritty of stem cell biology: on November 15, a session entitled “Endogenous stem cells and their niches” featured talks by Prof. Valentina Greco (Yale) and Prof. Sean Morrison (Texas Southwestern Medical Center), as well as a student presentation by Charlotte Sénéchal (McGill University). The following day, research by Dr. Tae-Hee Kim (SickKids and University of Toronto), Dr. Prisca Liberali (FMI Switzerland) and trainee Monique Marylin Alves de Almeida (University of Alberta) was highlighted in a session focused on “Stem cells, embryos and organoids.”
The two sessions showcased themes and approaches currently at the forefront of the stem cell field; specifically, both sessions focused on the topics of niches impacting stem cell behaviour, as well as the use of single-cell technologies to characterize niche cell properties. The importance of the stem cell niche (the niche being the collective term for the immediate environment of a stem cell, which encompasses different supportive cell types as well as matrix proteins and growth factors produced by these) cannot be overstated. First conceptually described in 1978, the niche is appreciated as an environment without which stem cells do not maintain their pluripotency, and appreciating niche properties is therefore crucial to our understanding of stem cell behaviour.
Prof. Morrison, for example, described recent work on characterizing the bone marrow stem cell niche, in which hematopoietic stem cells depend on the growth factors secreted by neighbouring sinusoidal and arteriolar niche cells. Wanting to understand the cellular organization of the bone marrow niche beyond this interaction, his group employed single-cell RNA sequencing (scRNA-Seq), a relatively novel and now widely popular technique that can identify cell populations in a tissue in an unbiased way, based on their distinctive gene transcription patterns. This led to the discovery of a sub-population of arteriolar cells that have been thus far underappreciated in their role as osteogenic progenitors and niche cells for lymphoid progenitors.
Also leveraging the power of scRNA-Seq, Prof. Kim investigates the stem cell niche in the gut, where an increasing number of mesenchymal cell types are being revealed and their roles in stem cell maintenance uncovered. Strikingly, his recent work is extending the definition of the stem cell-maintaining niche to include microbiota, whereby specific bacteria affect gut stem cell function by increasing the number of macrophages, which in turn affects stem cell differentiation.
Both talks showcased the importance of single-cell technologies to hunt for cell types that play important roles in defining the chemical and physical environment of stem cells. Beyond improving our understanding of stem cell biology, such studies may well result in novel therapeutic approaches aimed at protecting or directing stem cell fate in old age or disease.
While the niche is commonly appreciated as the “stem cell support network,” consisting of stromal cell types in various tissues, two talks at this year’s TMM showcased niches in a different, perhaps less appreciated, but wildly intriguing light.
Prof. Liberali spoke about what may be the first ever niche to develop within the developing embryo. This happens in an event termed “symmetry breaking,” when the first axis of body symmetry is established in the gastrula. Liberali’s question “How do initially genetically identical cells generate tissues with different properties?” required her group to devise a high-throughput in vitro system to analyze the process of gastrulation, where they could exploit scRNA-Seq and microscopy to establish spatio-temporal trajectories of gene expression along the timeline of the symmetry breaking process.
Specifically, cells at the centre of the gastrula, where they are exposed to a high concentration of the niche molecule fibronectin, express the developmental gene SOX2, while cells localized to the outer shell of the cell mass express the gene Brachyury, and a gradient of the molecule Wnt is established between the two zones. Overall, these different niches lead to elongation of the cell mass along one axis and cells belonging to core vs. outer shell were characterized by scRNA-Seq, thereby uncovering many more differences between the two stem cell populations and shedding light on this fundamental process.
Prof. Greco described her group’s efforts in understanding the niche of skin follicles in the context of epithelial tolerance to mutations. Not using scRNA-Seq, but instead stunning in vivo imaging of individual fluorescently labelled cells, Greco was able to visualize stem cell behaviour in the living mouse skin follicle and posed the question: given that the skin is an organ that dramatically reorganizes every day as old skin is shed, and given that mutations occur often in this tissue, how does the skin deal with the constant presence of a “mosaic of mutant cells?”
To answer this question, her group enhanced cell growth through Wnt signaling in mice, which led to tumour growth in the skin. Strikingly, however, over time these tumours simply disappeared, returning the tissue to normalcy. This phenomenon indicated to Greco that mutational errors are corrected within the skin niche through an unknown process of epithelial tolerance.
Further experiments showed whether tolerance occurs or not depends on the mutation, whether the epithelium is overall stimulated (e.g., by the presence of a wound) and that tolerance can be accomplished through a differential in proliferative capacity between mutated and non-mutated cells. Greco’s work describes a departure from the idea of niches solely as environments supporting growth and differentiation, and proposes dynamics of growth competition within the same niche, designed to keep mutated epithelial cells in check and eradicate damaged cells altogether.
The paramount importance of stem cell niches was reflected in this year’s TMM sessions. Thanks to such studies, as well as the power to analyze niche cells with unprecedented resolution, discoveries about how to maintain populations of stem cells or how to direct their differentiation are bound to emerge from this collective
Elisa D'Arcangelo
Latest posts by Elisa D'Arcangelo (see all)
- About tissue regeneration, fibroblasts and reindeer - December 27, 2021
- It’s all about the Niche! Take-aways and highlights from TMM 2021 - November 25, 2021
- “Good Enough” tissue engineering - May 11, 2021






Comments