 To mark World AIDS Day, I caught up with the International AIDS Society (IAS) 2021 HIV Cure and Gene Therapy Forum’s opening session, which focused on the intersection between HIV cure research and rapidly advancing gene-based therapies.
To mark World AIDS Day, I caught up with the International AIDS Society (IAS) 2021 HIV Cure and Gene Therapy Forum’s opening session, which focused on the intersection between HIV cure research and rapidly advancing gene-based therapies.
The session sought to address two increasingly urgent questions: How can advances in gene therapy from other fields be applied to developing an HIV cure? (The IAS definition is an intervention that would lead to durable, antiretroviral-free suppression of HIV). And secondly, how can an intervention be made available to all in need around the world.
I remember how rightly cautious my former colleagues at the UK’s National AIDS Trust were whenever the topic of a potential HIV cure came up. That was seven years ago and given the pace of progress in gene therapy, I wasn’t too surprised to find a markedly more optimistic tone in presentations at IAS 2021. “After several decades of effort and false starts, gene therapies now hold real promise to provide clinical benefit for the treatment of diseases that were previously untreatable, especially in the areas of oncology, hemoglobinopathies and rare diseases,” moderator Dr. Paula Cannon commented in her opening remarks.
Around 38 million people are living with HIV globally. Approximately 80 million people have become infected with HIV since the start of the epidemic, and 36 million lives have been lost to AIDS-related illnesses. Antiretroviral therapy (ART) has made huge progress. Thanks to ART, a person living with HIV now has a similar life expectancy to an HIV-negative person – providing they are diagnosed in good time, have access to quality medical care, and adhere to treatment. As of 2020, 27.5 million people are accessing ART for HIV and now that the condition can be effectively managed, researchers can pool their efforts in search of a cure. For that, many have their sights set on gene therapy.
Up until recently, ART was the only proven therapeutic response on the scene. This all changed with Timothy Ray Brown and his bone marrow transplant. In 2007, Timothy received an allogeneic bone marrow transplant for acute myeloid leukemia. Timothy was living in Berlin at the time and had HIV, but needed a bone marrow transplant because his leukemia didn’t respond to chemotherapy anymore.
Timothy’s doctor managed to find a donor who was a match for his bone marrow transplant and resistant to HIV because of a genetic mutation known as CCR5-delta 32, which hampers HIV’s ability to infiltrate immune cells. This mutation is responsible for the two types of natural HIV resistance that exist. One per cent of people descended from Northern Europeans (particularly Swedes) are homozygous carriers of this mutated gene (meaning they inherited a copy from both parents). These lucky few are thus immune to HIV infection.
Timothy stopped his HIV medication, and 20 months passed by without a viral rebound. These astonishing results were reported in the New England Journal of Medicine in 2009, and Timothy — who had not yet made his identity public — became known as “the Berlin patient.” When he realized what his story meant for people living with HIV, he came forward to use his unique voice and platform for advocacy.
Dr. Hans-Peter Kiem, a pioneer in the field of cell and gene therapy, was first to deliver a keynote presentation at the session. He opened by recounting Timothy’s story, explaining how it set the stage for the use of cell and gene therapy to enhance control and eradication of HIV. It was in part Timothy’s treatment success, after all, that led to the creation of The Martin Delaney Collaboratories for HIV Cure Research — the flagship NIH program on HIV cure research that launched in 2011. Dr. Kiem and Dr. Keith Jerome were awarded one of the first three collaboratories, which saw them launch “Defeat HIV,” with a mission to develop cell and gene therapies for an HIV cure — the only Collaboratory pursuing that line of investigation at the time.
In his presentation, Dr. Kiem outlined how CAR T-cell therapy could also have a role to play: “We’ve hypothesized that car T cells, just like in cancer setting, could also be used in the HIV setting. So we could have CAR T-cells directed against the HIV envelope, for example. And then this could replace this allogeneic effect that was likely also contributing to Timothy Ray Brown’s cure.”
Following Timothy’s cure came Adam Castillejo — aka the London patient —in 2019. Adam was the second person to be cured of HIV after receiving a bone marrow transplant to treat Hodgkin’s lymphoma from a donor who also had the CCR5-delta 32 mutation. These combined successes provided proof of principle that the key to a future HIV cure could lie in this genetic mutation. Against a backdrop of rapidly developing gene-editing technology such as CRISPR, this was an exciting development indeed.
In March 2019, Dr. Jerome Groopman wrote in the New Yorker, “[CRISPR] techniques may be used to genetically alter the cells of people with HIV, inactivating the CCR5 gene. Those people should then become resistant to the virus, and hopefully recapitulate the outcomes of the Berlin and London patients.”
Making sure low-income countries aren’t left behind
While these advancing gene therapy approaches are exciting, Dr. Kiem cautions that just like the bone marrow transplants that cured Timothy and Adam, they need to be developed in expensive, high-tech facilities. A growing concern is that gene therapy approaches will not be available to the millions of people living with HIV in places that need a cure the most — like in sub–Saharan Africa, which is home to two-thirds of people living with HIV.
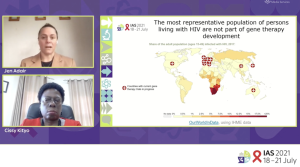 Dr. Kiem isn’t alone in this concern; in the dual keynote speech that followed, Drs. Jennifer Adair and Cissy Kityo outlined their efforts to bring cell and gene therapy interventions to resource-limited parts of the world.
Dr. Kiem isn’t alone in this concern; in the dual keynote speech that followed, Drs. Jennifer Adair and Cissy Kityo outlined their efforts to bring cell and gene therapy interventions to resource-limited parts of the world.
Kicking off the presentation, Dr. Adair revealed a map showing that, to date, low- and middle-income countries (LMIC) have been excluded from gene therapy development altogether. “If the standard case for HIV is not included in the development of a gene therapy that should eventually be used to treat those patients, we are probably setting ourselves up for failure,” Dr. Adair commented.
Following, Dr. Kityo emphasized the importance of learning lessons from the rollout of ART for HIV patients in Africa. She notes that it took 17 years from drug discovery to the therapy becoming widely available to patients in the region. “Advocacy was very central to access, even in the developed countries,” commented Dr. Kityo. “How can we use advocacy today? How can we make our voices louder through these mechanisms?”
As well as raising vital questions, Dr. Kityo and her team are already working on solutions. In late 2020 they launched the Global Gene Therapy Initiative (GGTI), an alliance of key stakeholders including clinicians, scientists, engineers, advocates, patients and caregivers brought together by the shared interest in enabling access and implementation of gene therapies as curative medicines in LMIC.
The impetus for the group’s formation was multi-faceted, but relied heavily on cold calls between individual stakeholders who immediately identified overlapping interests. Dr. Kityo revealed how she and Dr. Adair started working together: “I had been reading about Jen’s work, her goals and aspirations. I called her one morning. It was 1 am, and I explained what I wanted to do, and she immediately said ‘Yes,’ very emphatically.”
GGTI published its first report in Nature’s Gene Therapy in September this year, setting out its ambition of introducing two gene therapy Phase I clinical trials in two LMIC, Uganda and India, by 2024. The paper highlights how local labour could keep manufacturing costs down and avoid altogether the custodial and logistical assurance costs associated with central manufacturing, thus improving affordability.
Necessity is, after all, the mother of invention, and the solutions born out of the need to bring gene therapies to resource-limited parts of the world will surely benefit us all. As Dr. Adair put it, “I like to think that if we actually try to face the problems that we’d have to cross to get gene therapy in low- and middle-income countries, we would actually be advancing the technique in a way that could benefit patients everywhere.”
When it comes to a product profile for an accessible HIV cure, Dr. Adair says that ultimately we need to be able to treat and monitor patients with as inexpensive a platform as possible. “A single intervention, on an outpatient basis, this means simple and cheap diagnostics – a shot in the arm, single treatment. And affordable non-invasive monitoring. . . there is much that the gene therapy field can learn from and in partnership with the HIV field.”
Cal Strode
Latest posts by Cal Strode (see all)
- What’s in the mix for 2026: ARM’s State of the Industry briefing - January 28, 2026
- World AIDS Day: Update on HIV cure research and gene therapies - December 1, 2025
- Headwinds and tailwinds for cell and gene therapy under the second Trump administration - March 11, 2025






Comments