The 2021 Till & McCulloch Meetings (TMM) are where the heart is. More specifically, I am referring to the major advances in cardiovascular regenerative medicine presented during the Bioengineering Regeneration plenary session on the final day of the conference. It was my privilege to both attend and participate in the conference on behalf of Signals and I was quite amazed by the advances in such a challenging field.
The Bioengineering Regeneration session was chaired by Dr. Molly Shoichet, University of Toronto (UofT). Dr. Shoichet kicked off the session with a presentation on the state of bioengineering today. She discussed work by Dr. Derek Van der Kooy, (UofT), in retinal stem cells, Dr. Bill Stanford, Ottawa Hospital Research Institute, in drug responses in metastatic cells, and Dr. Penney Gilbert, (UofT), who is focusing on 3D culture models of healthy and injured muscle models, to name a few.
Dr. Jeffrey Hubbell, University of Chicago, discussed his work on modulation of growth factor binding and artificially prolonging interaction with their respective receptors. This allows for a more sustained signal in response to receptor binding instead of the rapid on/off switching seen in nature, key for enhancing the effect of a drug, for example.
Zongjie (Daniel) Wang, a 4th year PhD student in the lab of Dr. Shana Kelley, (UofT), presented his work focused on the intersection between regeneration, cancer and safety, as the cells used in regenerative medicine have properties found in cancer like constant growth and less differentiation. In fact, one in 4,000 induced pluripotent stem cells (iPSCs) has the potential to form teratomas. With Stem Cell Quantitative Cytometry (SCQC), Zongjie was able to assess iPSC populations in both a sensitive and rapid manner to detect and eliminate rare tumorigenic cells. SCQC represents a pathway forward towards the safest possible use of regenerative cell therapies.
The focus of my coverage is on the work of Dr. Milica Radisic, a Professor at the University of Toronto, Canada Research Chair, and a Senior Scientist at the Toronto General Research Institute. Her team’s research efforts have centred on developing new biomaterials for cardiac tissue repair/regeneration and I recap the recent developments presented at TMM 2021.
Cardiovascular regenerative medicine 101
Given the heart is largely recalcitrant towards regenerative processes, Dr. Radisic’s work involves significant hurdles, but also the potential to drastically alter the often-poor outcomes associated with cardiac events. An important theme throughout her talk was that better function follows better form. A system that encapsulates a good structure is necessary for any kind of regenerative process to occur.
Coupled with better designs, the standardization of iPSC culture protocols has facilitated advancements in cardiac regenerative treatments as it has become easier to harvest cardiomyocytes, the precursors of heart muscle tissue. However, proper support is needed for their survival and successful delivery to the heart.
One way to do this is by delivering them in scaffold structures made up of flexible polymers known as elastomers. Biomaterials such as 1,8-octanediol, poly(octamethylene maleate (anhydride) citrate) (POMaC) and citric acid, molecules found in humans, can be engineered with special moulds to produce functional scaffolds with desired properties. Given their elastic nature, these scaffolds are contractile and act similarly to heart tissue, overcoming a serious barrier within cardiac tissue engineering.
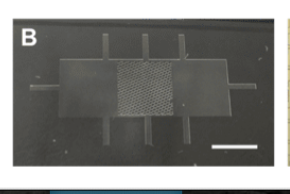
The two-component adhesive “Band-Aid-like” patch. Screen capture from Prof. Milica Radisic’s talk during TMM 2021.
The two-component adhesive patch
One of these scaffolds made from POMaC could allow cardiomyocyte delivery noninvasively, overcoming the need for invasive heart surgery. Dr. Radisic’s group was able to do this by designing the orientations of these polymers precisely, optimizing for proper folding so that, upon injection, through a keyhole incision, the scaffold properly expands and can affix to the heart. To ensure proper adhesion to the heart, a different “sticky” polymer was moulded around the scaffold in the centre to form a Band-Aid®-like patch. However, Dr. Radisic mentions that this is one example and there remains the potential to mix many different polymers together when designing a patch guided by the treatment goal.
The hook-in-tissue “Velcro®” approach
A sturdier strategy for anchoring scaffolds involves using a Velcro-like scaffold that affixes to live heart tissue. These scaffolds are made with robust materials capable of withstanding manipulation and contractility of the heart upon delivery. One application of these robust scaffolds recently involved using sheets of polymers with embedded cardiomyocytes that are then wrapped to form the tube-like shape of a ventricle. This model was then able to pump fluid upon stimulation, providing a powerful approach to rebuild ventricles damaged through scar tissue/heart failure.

The AngioChip connected to the vasculature. Screen capture from Prof. Milica Radisic’s talk during TMM 2021.
The AngioChip
The modular design of polymers allows researchers the ability to achieve the desired properties. For example, removing citric acid and using a 124 polymer in substitute yields a more malleable and elastic scaffold that better matches cardiac tissue properties. These polymers can then attain conductive properties through carbon nanotube addition, providing biomaterial that best mimics heart tissue.
With this inherent modularity in polymer design, Dr. Radisic’s group was able to develop a 3D scaffold that could mimic internal vasculature. The AngioChip utilizes a multi-polymer approach to develop a robust material that acts and responds to stimuli as functional blood vessels would. Fine-tuning of polymers also ensured that the mechanical properties of AngioChip fully matched that of native tissue.
A major advantage of AngioChip is it can be “hooked up directly to the plumbing,” explains Dr. Radisic, given the materials it is synthesized from. Importantly, clotting is also prevented, despite the AngioChip’s small size, due to its composition. This allows AngioChip to become a viable alternative to rebuilding damaged blood vessels and offers a simple method to re-establish blood flow within organs.
Itaconic acid – a polymer of the future
To continue these trailblazing developments, the materials used to make polymers and scaffolds will need constant interrogation and innovation, says Dr. Radisic. One molecule with exciting future potential is itaconic acid (ITA). ITA is a molecule critical in the innate immune response and its polymers are shown to have similar properties.
To further assess ITA’s properties as a polymer, Dr. Radisic’s team studied it in mice via injection to the peritoneal cavity. A first assessment involved looking at inflammation, a common side effect of placing biomaterials in the body. The level and persistence of inflammation ultimately determine implant integration success. Compared with silicone polymers, ITA-derived polymers produce negligible amounts of inflammation after 10 days while silicone polymers maintain inflammation indicating their viability for use in biomaterials.
ITA polymers also promote a regenerative environment. Resident macrophages are thought to be important for regeneration and are lost upon tissue damage. Re-establishing them is important to produce an environment favourable for healing, a property of ITA polymers. Coupled together, ITA-derived polymers can both integrate with minimal inflammation and promote a healing environment, highlighting their immense potential for use as a biomaterial.







Comments