In 2005, the Stem Cell Network inaugurated the Till & McCulloch Award to honour the important work of Canadian scientists Drs. James Till and Ernest McCulloch, for which the annual Till & McCulloch Meetings (TMM) are also named. This year’s TMM attendees had the pleasure of watching Dr. Till introduce the 2021 Drew Lyall Award and the Till & McCulloch Award session. He highlighted this year as a particularly special one for Canadian stem cell research: 2021 marks Dr. Till’s 90th birthday; it also represents 60 years since the publishing of the first of his three seminal papers on colony forming bone marrow cells, which would then become known as stem cells; and it has been 20 years since the inception of the Stem Cell Network and 10 years from the beginning of these meetings under the present name.
Congratulations to the Drew Lyall Award winner, Darren Miquel Blackburn (Lady Davis Institute for Medical Research, McGill University), who spoke about his work in defining the properties of the muscle stem cell niche.
His talk was followed by Dr. Jeff Biernaskie (University of Calgary), winner of this year’s Till & McCulloch Award. His lecture was, in this writer’s opinion, exceptionally exciting.
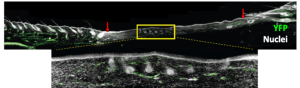
Screen capture from Dr. Jeff Biernaskie’s talk at TMM2021. Skin wound on a mouse’s back showing regenerative central region with new hair follicles. The wound lies in between the two red arrows.
Dr. Biernaskie presented a scientific story that started out with a fundamental question, which is well known to experts in the field of tissue regeneration, but which has not found satisfactory answers thus far: Why can certain tissues, after being wounded, regenerate, while other tissues form scars? While seemingly narrow at first, the implications of this question are far-reaching.
Fibrosis (scarring) is a function-impairing process that can affect most organs in our body (nearly 45 per cent of deaths are estimated to arise from fibrosis in the developed world) and thus knowledge on how to lessen a fibrotic response could be truly transformative to health care across disciplines. Moreover, researchers have been dreaming about regenerating tissues to full form and function for decades. While images of salamanders with newly growing limbs jump to mind, Dr. Biernaskie’s talk showed that this field is inching slowly, but surely, away from amphibians and more towards us mammals.
It is known that fibroblasts – spindle-shaped cells that are present, in some version, in just about every tissue in our body and are responsible for maintaining tissue integrity – play roles in both regenerative processes as well as fibrotic ones. The jumping off point for Dr. Biernaskie’s work was to understand what induces fibroblasts in a wounded tissue to engage one rather than the other path.
To conduct this study, his group needed a method to examine both processes in an analogous environment. The initial model he used encompassed a setup whereby mice were given a large wound on their back skin, which, upon healing, formed two distinct zones: at the wound periphery, fibrosis dominates and leads to scar tissue and a lack of everything else – no hair growth, no skin glands, no pigmentation.
At the wound’s centre, however, a regenerative region is established where normal skin components reappear over time. As the group has reported in their 2020 Cell Stem Cell paper, when fibroblasts were isolated from both tissue regions and analyzed at the transcriptome level with single-cell resolution, it became clear that these cells are radically different. Only fibroblasts at the wound’s centre appear to have regenerative competence, expressing genes important for skin developmental programs, while peripheral fibroblasts lack this regenerative property and instead express the to-be-expected fibrotic genes typical for scarring.
To investigate why regenerative capacity was observed exclusively in a spatially distinct pool of fibroblasts at the wound centre, Dr. Biernaskie’s group turned to another animal model: the reindeer. This is because reindeer antlers are covered in a layer of specialized tissue termed velvet that regenerates without scarring every year when antlers are shed and re-grow, making this a beautiful model of regeneration in mammals.
By comparing fibroblasts in velvet tissue (the regenerative setting) to those in reindeer back skin, which scars but does not regenerate, both before and at different time points after injury, specific patterns were uncovered. Firstly, even before looking at injury, fibroblasts in back skin appear to already express gene programs that prime the tissue for inflammation, while velvet fibroblasts do not. Secondly, upon injury, these same pro-inflammatory fibroblasts acquire a phenotype that is prone to scarring and this phenotype persists over time. In contrast, velvet fibroblasts acquire the same phenotype initially but – and this is the kicker – they revert back to their regenerative selves halfway through the healing process. Thirdly, when the immune cell content in both types of wounds was examined, Dr. Biernaskie could see that the immune response in injured velvet abates over time, but it does not in back skin. So, what is the connection between these pro-inflammatory fibroblasts and a prolonged immune response? The link is provided by a specific type of inflammatory cell called the neutrophil, which matures quicker in the long-lived, pro-inflammatory niche provided by back skin fibroblasts. Unfortunately, these mature neutrophils then contribute to an environment that leads to scarring, rather than regeneration.
Overall, this work beautifully offers evidence to make the case that a niche provided by fibroblasts (and inflammatory cells) dictates the regenerative competence of a tissue with such determinism that injuries heal completely in one case, but are replaced by a scar in the other. Current work in Dr. Biernaskie’s lab focuses on obtaining control over whether a niche is scarring or regenerative by adding various signaling factors.
Most intriguingly for us humans, however, is the fact that the gene expression signature of velvet fibroblasts resembles that of human fetal skin, while back skin fibroblasts bear resemblance to human adult skin, implying that the rules that govern regeneration vs scarring in reindeer also apply to us and may in time be unlocked to eliminate fibrosis and scarring in humans.
Elisa D'Arcangelo
Latest posts by Elisa D'Arcangelo (see all)
- About tissue regeneration, fibroblasts and reindeer - December 27, 2021
- It’s all about the Niche! Take-aways and highlights from TMM 2021 - November 25, 2021
- “Good Enough” tissue engineering - May 11, 2021






Comments