
The panel on talent at ATW2022. L-R Angela Justice, Sharon Brownlow, Jill Elliott and Lynn Fischer, CEO Title 21 Health Solutions
People and patients were top of mind at Advanced Therapies Week 2022 (ATW2022) in Miami Beach, Florida, which shouldn’t come as a surprise to anyone in the industry or following the industry.
Talent is a big issue right now. Good people are moving around and replacing them is difficult. It’s a challenge to fill manager-level positions and above, and with the growth of manufacturing finding employees trained in Good Manufacturing Practices (GMP) is an effort.
The panel discussing “Workforce of the Future: Talent, Change Management and Enabling Future Development” offered valuable insight into these issues as well as solutions. The first three speakers, Dr. Angela Justice, Chief People Officer at TCR2 Therapeutics, Sharon Brownlow, Chief Business Officer, Cell and Gene Therapy Catapult, and Jill Elliott, Vice President, Cell and Gene Therapy Commercialization at Novo Nordisk, all acknowledged the talent shortage we’re experiencing right now.
Dr. Justice noted that exponential growth in the pipeline means we need the same growth in talent. Her talk focused on how to retain your employees: the importance of scaling your culture as you grow; anchoring your culture through your values and framing values as competencies; and, finding your “force multipliers” – whether that is technology or people, you have to invest in them to amplify their results.
Ms. Brownlow spoke about the skills gap we are seeing in the industry and shared that a 2021 Catapult survey predicted an increase in sector jobs from 7,000 to 15,000 by 2026. The situation in Canada may be even more dire. Dr. Jillian Murray with Mitacs writes “Canada is already facing a huge talent shortage for existing infrastructure, let alone recent investments and commitments [$2.2B over seven years toward Canada’s Biomanufacturing and Life Sciences Strategy] to build more in the future. BioTalent Canada’s 2021 National Report predicts that Canada’s bioeconomy needs 65,000 jobs by 2029 — this includes 16,140 biomanufacturing workers and 5,160 in bio-health manufacturing alone. At this pace, it is predicted only 25% of the available roles will be filled.”
The industry is making strides to address these issues by developing training programs; for example, the UK’s Cell and Gene Therapy Catapult announced in July 2020 it had government funding to develop national Centres for Advanced Therapies Training and Skills (nCATTS), and CellCAN and CCRM announced its own training institute – the Canadian Advanced Therapies Training Institute – last summer. But there’s wide recognition that, collectively, our sector needs to go even further to meet the burgeoning demand.
One suggestion Ms. Brownlow made for tackling the skills gap is to hire and train workers already employed in sectors where good manufacturing practices are required (for example, food and wine). Catapult has a “career converter” to help workers identify what skills they currently have that are transferable to the cell and gene therapy industry. Catapult is already making great inroads in training: its ambition is to be the “go-to” place for everything related to education and training, as illustrated in this slide.

Slide presented by Sharon Brownlow, Chief Business Officer, Cell and Gene Therapy Catapult, at ATW2022
Jill Elliott stated that it’s a candidate’s market right now, and after putting in the effort to find new staff the challenge is to keep them (see slide 1). But she also recognizes that not all employees can adjust and handle the pressure of an industry that requires a great deal of passion for problem-solving and resilience. Below are some competencies Novo Nordisk seeks in employees (slide 2).
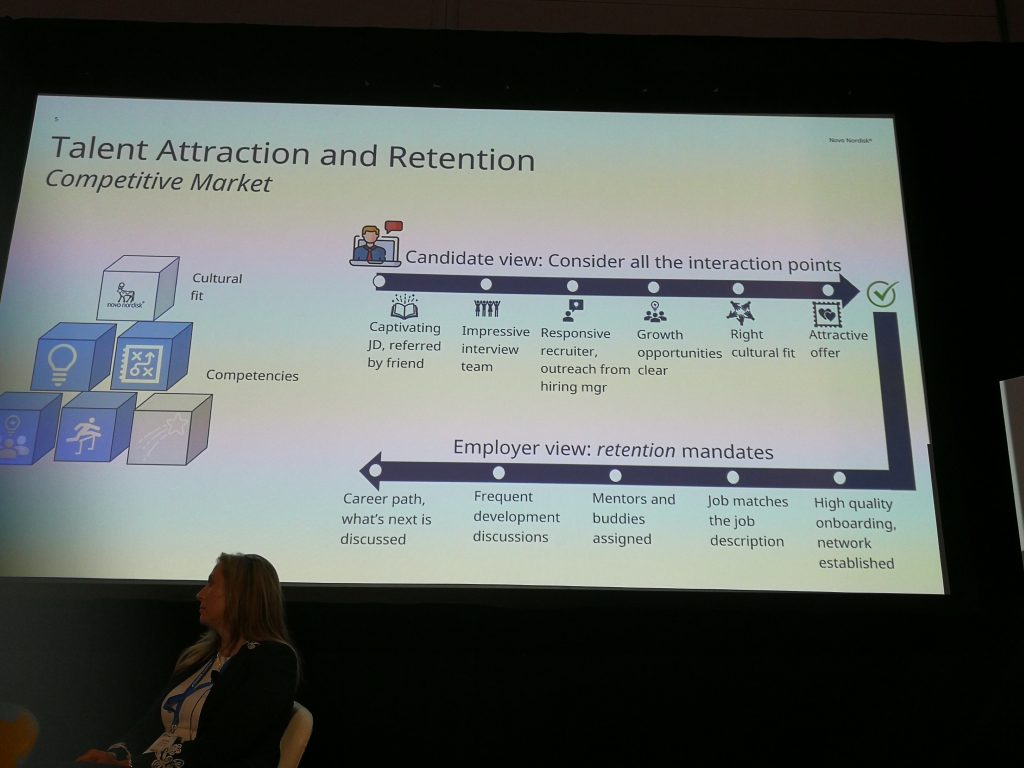
Slide (1) presented by Jill Elliott, Vice President, Cell and Gene Therapy Commercialization at Novo Nordisk, ATW2022

Slide (2) presented by Jill Elliott, Vice President, Cell and Gene Therapy Commercialization at Novo Nordisk, ATW2022
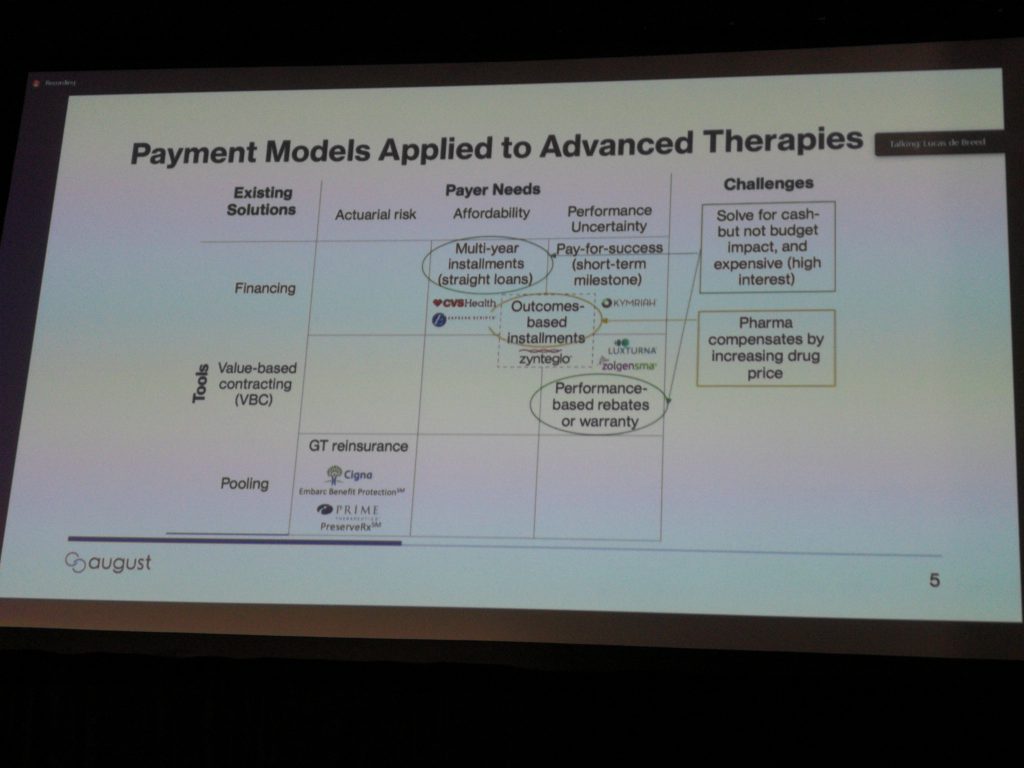
When it comes to reimbursement, there will be no “one-size-fits-all” solution. One reason why the industry is struggling with reimbursement is that our payer landscapes differ between countries. A country with (free) universal health care, such as Canada, will have different challenges than a country like the United States with public and private health insurance. An unprecedented sea change is coming to the sector, with a shift from chronic diseases, which require long-term treatment and thus payments spread out over time, to curative cell and gene therapies that expect very large payments upfront.
Models currently being used and considered are: treatment milestones – some upfront payments are made and a portion is returned by the developer if milestones aren’t reached; subscription-based – plan members pay a fixed fee for unlimited access to certain therapies, as needed. See the slide below for more details.
At “Patient and Payer Challenges: Enabling Access to Patients through Market Access, Pricing and Patient-Led Discussion,” we heard from Lucas De Breed, Managing Director, August Care, John Schick, Vice President, Global Market Access, InstilBio, Clark Paramore, Head of Value Determination, Vice President of HEOR, bluebird bio, and Tom Whitehead, Founder, Emily Whitehead Foundation.
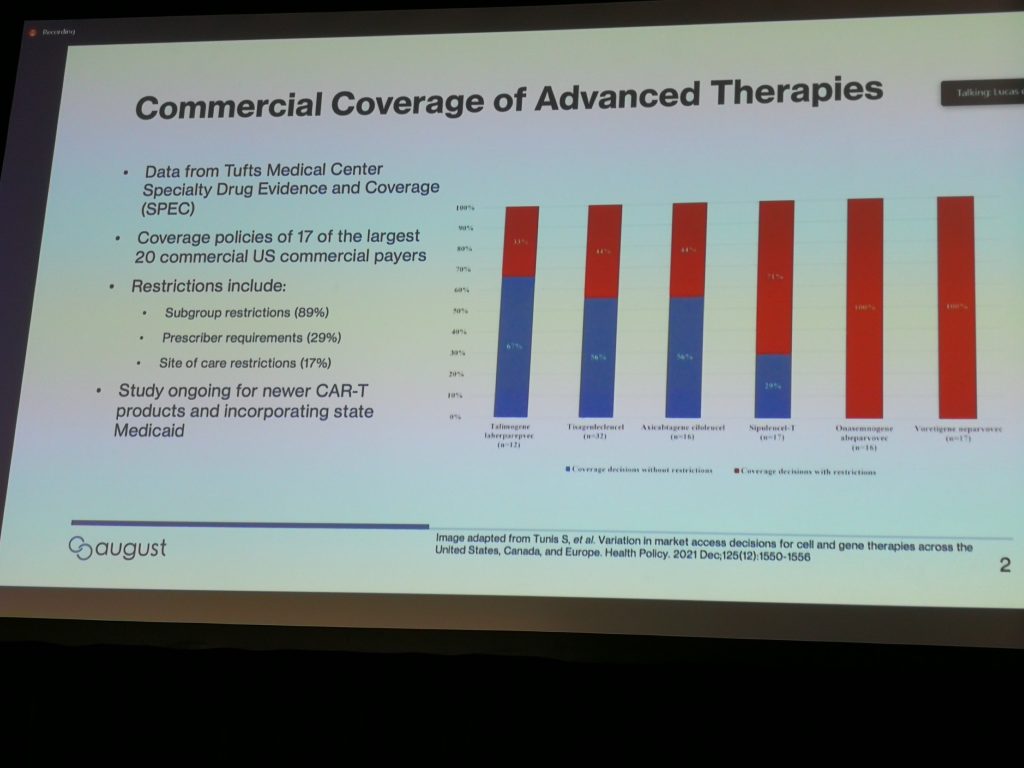
This slide from Lucas De Breed shows progressively poorer coverage, based upon the number of restrictions issued, from different U.S. payers (the red bars in the image) for different indications. The data in the slide come from this study that found health plans applied restrictions inconsistently. According to the authors, “This variation is important because US residents who are enrolled in different health plans will not have equal access to these therapies, even when the clinical circumstances are identical.” Obviously that’s a problem.
John Schick stated that FDA approval is a key driver of coverage and payers establish coverage criteria. The decision-makers are (from most influential to least): payers, hospital CEO/CFO, centre director, centre medical director, attending physicians and external societies. The lessons learned from CAR-Ts show that coverage decisions were based on clinical need, while reimbursement was mostly decided based on finances due to the cost burden on centres. Mr. Schick suggested that if there is no reimbursement methodology that fits a particular indication, it is incumbent upon us to educate and start negotiating for it. He stated that payers don’t necessarily want to deny coverage, but sometimes they don’t know how to offer coverage within their routine reimbursement processes and that’s why education is needed.
Bluebird bio is building an evidence case for coverage of one-time therapies to educate payers. Clark Paramore gave the example of diabetes treatment costs that have increased 74 per cent since 1999 due to new innovations. The “one and done” cell or gene therapy will reduce long-term costs but it’s a new paradigm for payers: these products will last a lifetime; therefore, they are priced well. Bluebird has built a mobile application for patients to collect data to show the burden of their disease on a daily basis.
I also attended an interesting roundtable discussion hosted by Michael Griffin, Product Manager, Global Commercial Development, Cook Myosite. Titled “Treatment Development Paradigm for Quality of Life Conditions: Regulatory Considerations & Collaborative Ecosystem,” we discussed whether “Quality of Life” (QoL) should be renamed in the context of reimbursement for non-life-threatening illnesses. Does the name reduce their importance? Does the term suggest there isn’t an immediate need? We didn’t reach a consensus, but you are encouraged to leave your thoughts in the comments below.
As we heard, QoL – or life-altering – conditions are harder to measure, and there is no benchmark for the FDA to regulate. It’s too difficult to quantify the emotional distress that might accompany one of these indications (for example, urinary incontinence) but these treatments can’t be dismissed: there is a belief that, in the future, most therapeutics will be QoL indications.
Insurers currently balk at the price tags of QoL treatments and see these costs as a drain on the economy as patients live longer, but potentially unhealthy or unproductive lives. While regulators are seeing the value, insurers are skeptical. This is where the need for education, as Clark Paramore discussed, comes in. The FDA and payers may be paying attention to the patient advocates, but they will make decisions based on the science. As I mentioned above, emotional distress can’t be measured, but time and opportunity costs can be. If a patient spends 10 hours per week dealing with her condition, that translates into data. Developers would be wise to leverage patient advocacy groups to build education and awareness to influence regulators and payers.
If you missed my first blog on Advanced Therapies Week 2022, catch up here.
Stacey Johnson
Latest posts by Stacey Johnson (see all)
- Right Turn: Top 10 blogs from 2025 - January 9, 2026
- Right Turn: Season’s greetings and upcoming event - December 25, 2025
- Right Turn: Stem cell supplements: A growing market with growing risks - December 19, 2025






Comments