We (or maybe it’s just me) talk about cancer immunotherapy around here A LOT. It’s hard not to when there are so many new advancements in this field of cancer research! I thought it was time to provide a guide to cancer immunotherapy, highlighting some of the more common treatments being used today.
What is “cancer immunotherapy?” The Cancer Research Institute has a great definition: “cancer immunotherapy, also known as immune-oncology, is a form of cancer treatment that uses the power of the body’s own immune system to prevent, control, and eliminate cancer.” Our immune system is powerful, and great at recognizing “foreign” entities and destroying them. However, as I’ve covered in previous posts (here and here), cancer cells continue to find new ways to evade the immune system. Most cancer immunotherapies are designed to give the body a new edge over these adaptations.
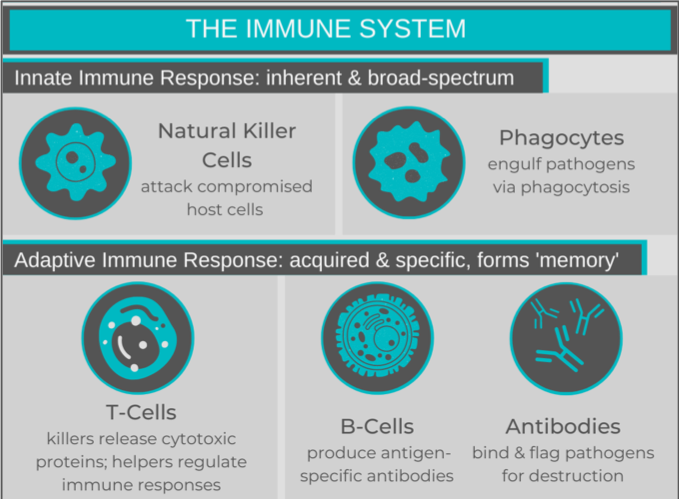
Before delving into immunotherapies, reviewing a couple of key concepts about “regular” immune system functions would be helpful. The immune system is typically seen as having two parts: the innate and the adaptive immune responses. The innate immune system is broad-spectrum response to a collection of stimuli that the human body has evolved to consider harmful, such as bacteria. Natural killer cells and phagocytes perform surveillance and destroy compromised host cells and pathogens, respectively. These cells activate the adaptive response by “presenting” processed fragments (antigens) of a pathogen or diseased cell to the adaptive cells.
The adaptive immune system is a specific response that develops after an individual’s exposure to a specific harmful stimulus. Activated T cells (1) release cytotoxic proteins, destroying their target cells (cytotoxic T cells) or (2) release signalling molecules to regulate the immune response (helper T cells). B cells recognize a single antigen, and produce antibodies once they are activated. The circulating antibodies bind their antigen, flagging the pathogen for destruction. After the triggering antigen disappears, some of the T and B cells become “memory cells” and remain in circulation to provide ongoing surveillance, allowing for a faster immune response in subsequent exposures. Just like our mental memories, everyone’s immunological memory is a little different, depending on our pathogen exposures (i.e. “experiences”). Similarly, vaccines allow for adaptive immunity to develop, without the infection.
So, now on to the good stuff!
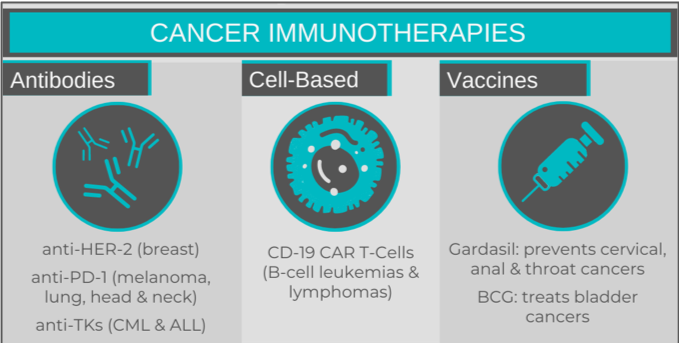
I like to think about cancer immunotherapies in the following categories: antibodies, cell-based therapy and vaccines.
Antibody immunotherapy
Cancer immunotherapy antibodies bind specific surface proteins on cancer cells, with the end result depending upon the target. Some antibodies interfere with a cancer cell’s ability to evade the immune system, while others will inhibit processes vital to a cell’s survival. An example of the former would be antibodies targeting PD-1, a receptor found on T cells. Antibodies bind the receptor instead of the usual ligand PDL-1, which is expressed in high levels on certain cancer cells. This prevents the cancer cells from being able to suppress the immune system through PD-1. The first anti-PD-1 antibody – Keytruda (pembrolizumab) – was approved in 2014 for treatment of melanoma. It, and others, are now being used in non-small cell lung cancers, head and neck squamous cell carcinomas, and Hodgkin’s lymphoma.
Familiar examples of process-inhibiting antibodies would be Herceptin (trastuzumab) and Gleevec (imatinib). Herceptin, commonly used in HER2-positive breast cancer, binds the HER-2 (human epidermal growth factor) receptor, preventing activation of signalling pathways critical for cell division. Other anti-HER-2 receptor antibodies exist, including some that are conjugated to chemotherapy drugs, making them “deliverable” to the tumour. Gleevec targets tyrosine kinase enzymes, which are essential for promoting cell division, and are permanently “on” in cancers such as chronic myeloid leukemia and Philadelphia-chromosome positive acute lymphoblastic leukemia (Ph+ALL). Blocking this enzyme prevents cancer cell proliferation.
These antibodies, despite the selective choice of their targets to proteins highly expressed on cancer cells, will also bind the same proteins on healthy cells. This leads to the death of healthy tissue, a similar side-effect to chemotherapy, but less widespread.
Cell-based immunotherapies
The most well-known cell-based therapy would be chimeric antigen receptor T cells (CAR T cells). CAR T cells are T cells (typically cytotoxic T cells) that have been genetically modified to fuse a tumour-recognizing extracellular receptor with the intracellular components of the T cell receptor. When a CAR T cell binds a tumour cell, the T cell’s cytotoxic immune response is triggered. I wrote about CAR T cells in detail in a previous post or read this post by Erika Siren.
Currently, CAR T cells are approved for the treatment of several hematological malignancies: B cell ALL and non-Hodgkin lymphoma, as well as multiple myeloma. For these conditions, a CD19-recognizing receptor is fused to the intracellular T-cell receptor. CD19 is a cell surface marker unique to B cells, and the anti-CD19 CAR T cells use this as a way to target the B cells that are responsible for the malignancy. These CAR T cells would be unable to differentiate between healthy and cancerous B cells, which is an unfortunate by-product.
Another limitation of autologous CAR T cells – made and reintroduced into the same patient – is their scalability. Unpredictable expansion from starting material varies from patient to patient and the genetic modification to insert a CAR is not always an efficient process, which can make it challenging to achieve the number of CAR T cells for a successful therapeutic product. To address this issue, the Centre for Advanced Therapeutic Cell Technologies at CCRM has demonstrated success with CAR T client projects in the past and continues to work to improve scale-up of these therapies, with a current focus on autologous CAR T but a genuine interest to expand that work into the allogeneic space in the near future.
Cancer vaccines
Just like vaccines for infectious diseases like measles or COVID-19, cancer vaccines work to prime a patient’s adaptive immune system. Gardasil is a well-known vaccine that is used to prevent infection of the cancer-causing human papilloma virus (HPV). Infection with HPV can lead to cervical, vulvar, vaginal, anal and throat cancers. Gardasil vaccines contain pieces of HPV proteins that prime the adaptive immune response and establish immunity, which will destroy any future HPV-infected cells, preventing tumour growth.
Other cancer vaccines do not prevent cancer from occurring, but rather prime the immune system to target an existing tumour, preventing disease progression. The first cancer vaccine ever used was for the treatment of early-stage bladder cancer. Bacillus Calmette-Guerin (BCG) – a vaccine originally for tuberculosis – is effective at preventing the growth and relapse of early bladder cancers, when delivered directly into the bladder (rather than by injection). No one really understands why BCG works in bladder cancer, as it is not caused by tuberculosis infection.
Lineage Cell Therapeutics is developing a new vaccine platform called VAC2, which aims to stimulate an immune response to human telomerase reverse transcriptase (hTERT). High levels of this enzyme are found in cancer cells, compared to healthy adult cells. hTERT preserves the integrity of DNA during cell division (which normally degrades over time), delaying apoptosis (cell death), essentially making cancer cells “immortal.” Currently, the company is testing their VAC2 vaccine in advanced non-small cell lung cancer patients in a Phase 1 clinical trial. After establishing vaccine safety, they hope to demonstrate that the vaccine is capable of inducing an immune response that decreases tumour burden and prevents metastasis in advanced lung cancer. The eventual goal would be to expand this to other cancers with high-levels of hTERT expression.
Unfortunately, with the exception of Gardasil, current cancer vaccines do not prevent cancer from occurring, but rather reduce existing disease burden.
As with traditional cancer therapies like chemotherapy and radiation, immunotherapies are not without their limitations. However, many of these immunotherapies are used in conjunction with other treatment modalities, increasing overall treatment efficacy. You’ve also likely noticed that many of the immunotherapies are being used in patients with advanced disease, which is often resistant to other treatments. These patients now have new treatment options with prolonged survival. Cancer immunotherapies are a great addition to the anti-cancer armamentarium, and continue to be an area of constant (and exciting!) development.
Sara M. Nolte
Latest posts by Sara M. Nolte (see all)
- Ottawa researchers make tumour-marker negative cancers positive with oncolytic viruses - December 19, 2024
- Cell-based therapy approved as alternative to standard UCB transplant for hematological malignancies - June 21, 2023
- Finding the perfect match: breaking down the science of organ and stem cell transplant matching - April 18, 2023






Comments