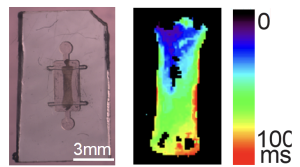
Optical mapping of electric activity in engineered tissues containing AVNLPCs generated by the Protze lab in collaboration with the Vasconcelos and Nanthakumar labs at UHN.
My heart is pounding, and my islets are secreting. Maybe it’s my fifth cup of sugary coffee kicking in, or maybe they’ve been inspired by the plenary session I’m recalling. While the former is more likely, I toy with the idea of the latter. In the “Clinical Advancement of Regenerative Therapies” session, at the recent Till and McCulloch Meetings, stem cell therapies for cardiac diseases and diabetes took centre stage.
Healing the heart
Dr. Stephanie Protze (University Health Network and University of Toronto) kicked off the session with her innovative work on developing “biological pacemakers” to treat cardiac arrhythmias. Dr. Protze’s research aims to replace the need for electronic pacemakers – which present several limitations, including high complication rates and a lack of autonomic responsiveness – with a biologic alternative made up of living cells.
In her talk, Dr. Protze presented her team’s progress in generating a cell replacement therapy for the atrioventricular node, the structure that conducts electrical impulses from the atria to the ventricles and whose failure is responsible for approximately 50 per cent of electronic pacemaker implants. Building on her previous work, Dr. Protze’s lab has pioneered novel methods to generate atrioventricular node-like pacemaker cells (AVNLPCs) from pluripotent stem cells. In a series of functional in vitro experiments, Dr. Protze verified that the lab-grown AVNLPCs displayed action potentials, beat rates, and conduction properties characteristic of the atrioventricular node.
After confirming functionality of the AVNLPCs in vitro, Dr. Protze’s team proceeded to test the cells in a guinea pig model. Three-dimensional tissues containing AVNLPCs were implanted within the heart and the cells remained viable and functional out to two weeks post-implantation. However, using dual-colour optical mapping with voltage-sensitive dyes to track both host tissue and graft tissue electrical signals, the team found that the beating rate of the implanted tissue was out of sync with the host for the majority of animals tested. Thus, the team is planning subsequent experiments where they will explore alternative methods to better integrate the AVNLPCs with the host tissues. Ultimately, Dr. Protze’s research could lead to new treatments for cardiac arrhythmias that restore physiologic conduction in the heart.
In keeping with the cardiac theme, the second talk of the plenary session, by Lin Wei (Henry) Tung, focused on regenerative mechanisms in ischemic heart disease, a condition arising from myocardial infarction that reduces cardiac perfusion and is a leading cause of mortality. Tung, a PhD Candidate in Dr. Fabio Rossi’s lab at the University of British Columbia, presented his work evaluating the role of the cardiac stroma in revascularization of fibrotic scars in the ischemic heart.
Through lineage-tracing experiments, immunofluorescence, and single cell RNA-sequencing, Tung reported that fibroblasts and mural cells (including both vascular smooth muscle cells and pericytes) represent distinct and mutually exclusive populations in the cardiac stroma under homeostatic conditions and in a mouse model for cardiac infarction. Intriguingly, he also uncovered a rare population of pericytes with a distinct gene expression profile and, after injury, these cells appeared to enter an “activated” state associated with upregulation of both fibrogenic and angiogenic genes.
To probe this further, Tung integrated spatial and single cell RNA-sequencing to investigate cell signalling pathways underlying the fibrotic scar after myocardial infarction. He presented regionally-specific signalling zones in which the infarct region displayed strong upregulation of fibrogenic signals, while the surrounding region (termed the “injured region”) consisted primarily of mural cells that seem to act in a compensatory manner by upregulating angiogenic signals. Interestingly, Tung’s “activated pericytes” appeared to localize to a border region between these zones where both fibrogenic and angiogenic signalling were upregulated. Altogether, Tung’s work helps to advance understanding of the spatially organized communication networks in the cardiac stroma that are activated to revascularize fibrotic scars following myocardial infarction. These results could aid in development of new therapies to improve revascularization for ischemic heart disease.
Seeking a cure for diabetes
The next speaker turned the spotlight toward the pancreas: Dr. Mattias Hansson presented on work at Novo Nordisk to produce high-quality islets from embryonic stem cells at large scales for transplantation in diabetes. Recognizing that stem cells are a renewable cell source that could circumvent a shortage of donor islets, the company created a process whereby embryonic stem cells could be derived, expanded and differentiated into beta cells – all under good manufacturing practice (GMP)-compliant conditions, which are required for eventual clinical application.
Dr. Hansson reported that the team can robustly generate beta cell clusters under scalable bioreactor conditions. Novo Nordisk has employed a range of in vitro assays for quality testing of the stem cell-derived islets, including flow cytometry, RNA-sequencing, and functional assays to demonstrate their glucose sensitivity and capacity for insulin release. Dr. Hansson also presented data using an immunodeficient mouse model for diabetes to show that transplanted human stem cell-derived islets persisted out to eight weeks post-transplantation and could normalize blood glucose levels in vivo.
While Novo Nordisk is able to generate large numbers of high-quality and clinical-grade islets, Dr. Hansson acknowledged that this is a starting point. The company is investigating strategies that could pave the way to a curative treatment for type 1 diabetes, including through the use of encapsulation devices that could help protect transplanted islets from immune reactions, and the use of hypoimmunogenic islets that are resistant to immune rejection.
The final speaker of the plenary session was Dr. Francis Lynn (University of British Columbia) who is working to understand maturation of pancreatic beta cells to improve outcomes in stem cell-derived islet transplantation. Dr. Lynn recapped findings from the recent ViaCyte Phase I/II clinical trial, noting that while results were promising overall, there was considerable heterogeneity in patient responses. Given that the devices used in these trials contained cells that were not fully differentiated, Dr. Lynn believes that implanting more mature and fully functional cells may achieve better outcomes for patients, and that gene editing approaches can be used to mitigate immune responses to the cells.
To get at this problem, Dr. Lynn presented a multi-pronged approach. His lab has employed methods to differentiate human embryonic stem cells into more mature pancreatic beta cells that the lab is able to gene edit for a variety of targets and with various reporters. The lab has also generated a reporter cell line based on expression of the mature beta cell marker islet amyloid polypeptide (IAPP) to track maturation of beta cells over time during in vitro differentiation, a tool that Dr. Lynn stated will have utility in screening experiments for beta cell maturation protocols.
Separately, Dr. Lynn presented recently published work aimed at understanding beta cell differentiation during in vivo embryonic development. The study authors established a transgenic mouse model termed “Ins1-GFP; Timer” that affords both spatial and temporal resolution to study beta cell neogenesis, providing novel insights into newborn beta cell niches. Ultimately, the lab is using their combination of tools to better understand how beta cells mature from early- to late-stage cells, in order to drive more efficient function of stem cell-derived islet therapies for diabetes.
Commentary: While the target organs discussed in this plenary session were disparate, for me, these talks reinforced the importance of understanding fundamental biological and developmental mechanisms to engineer effective stem cell therapies. A strong comprehension of (patho)physiological mechanisms will help to better define the problem and potential solutions in the form of stem cell treatments.
Kevin Robb
Latest posts by Kevin Robb (see all)
- Mesenchymal stromal cell product joins FDA’s list of approved cell and gene therapies - May 22, 2025
- Lessons learned from market-approved mesenchymal stromal cell products - August 22, 2024
- A bench-to-bedside story of BlueRock’s investigational stem cell therapy for Parkinson’s disease - November 9, 2023






Comments