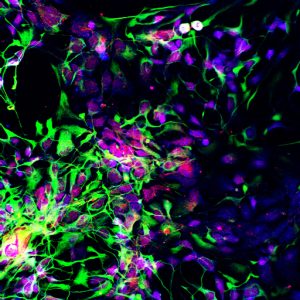
Motor neuron progenitors (green) derived from human embryonic stem cells. Such neurons could be used to treat spinal cord injuries and diseases of the motor neurons. This photo was taken by Sharyn Rossi in the lab of Hans Keirstead at the University of California, Irvine. Learn more about CIRM-funded stem cell research: www.cirm.ca.gov
Spinal cord injury (SCI) seemed like a logical first target for pluripotent stem cell therapy. In many cases a discrete lesion blocks the brain’s nerve signals from reaching the muscles or other tissues they are supposed to instruct. The solution: turn stem cells into the desired repair tool—in this case oligodendrocyte progenitors—and inject them hoping they will fill in the gap in the spinal cord.
Fourteen years after beginning the first clinical trial it has proven a tough target to hit. That’s in part because of two issues that frequently slow progress in regenerative medicine: developing and deploying new technologies, and not fully understanding the fundamental developmental biology of the target.
During a recent webinar by the publication The Scientist, two researchers presented work addressing both issues. Stephanie Willerth, University of Victoria, discussed using induced Pluripotent Stem Cells (iPSCs) and 3D bioprinting to make better models of various spinal lesions and how to do it a lot more efficiently. Her work, funded by the Government of Canada’s New Frontiers in Research Fund, is part of a moonshot consortium of 12 institutions in five countries called “Mend the Gap.” The second speaker, Nisha Iyer, Tufts University, defined the genetic signals that lead to various sections of the spinal cord.
That first spinal cord injury clinical trial began in October 2010, two years after I arrived to direct communications at the California Institute for Regenerative Medicine (CIRM). Expectations were high. The voters in California had approved a US$3 billion referendum to advance embryonic stem cell research three years earlier. Much of the public expected the work to produce cures within a decade and time was ticking. So when Geron Corporation announced it had treated the first person with cells derived from embryonic stem cells, the mood at the agency was over-the-top jubilation. (Although we did wish the first patient had been in California, not Georgia.)
The following year, our board approved a US$25 million loan to Geron for developing clinical trial centres in California. In September 2011, Richard Lajara became the first person to be treated with cells created from embryonic stem cells in the state. When a team at Stanford injected the cells, he became the fourth patient to receive the therapy. After one more patient was treated, in November that year, Geron announced it was discontinuing the trial. The staff at CIRM was devastated, as were the patients.
Geron said the only reason was financial: they wanted to concentrate on their recently approved cancer therapy candidate. And, the clinical trial did eventually continue when the company sold its stem cell assets to Asterias Biotherapeutics, which later merged with BioTime, Inc.
I recently talked to Richard about his mood then and now. All of the first five patients had injuries in the thoracic section of the spine, where the functional area is much wider and harder to treat than other areas, but also considered safer for a first-ever Phase I safety trial. Patients who had some use of their upper limbs, as Richard does, would be less likely to lose that function if something went wrong with the cells.
He said that when he was first injured there was more hope for a fast cure and that “Geron closing the trial felt like a major blow.” But he still has hope, perhaps more realistic hope, and has the chance to see the many advances in the field from his role on the CIRM oversight board, which he joined in 2021.
“I’m very optimistic for the field. I don’t think anybody anticipated it would take this long. Maybe not scientists, but the American people expect more cures on the table, but as you peel back the layers of the onion we didn’t realize how complex these diseases are. When You really look at the field there are more advances than people think. They are making great strides. Something is always on the horizon.”
Dr. Willerth agreed that there are a lot of layers to the onion of treating spinal cord injury. “The brain and spinal cord,” she noted, “are very complex organs and require a lot of effort to restore function after injury.” The differentiation protocols that turn iPSCs into mini-spines, or organoids, can take weeks or months, and it can be hard to keep the cells viable during the process. Dr. Willerth’s bioink, which she makes with a company she founded, Axolotl Biosciences, is good at nurturing cells and keeping them healthy as they become the desired tissue.
Her lab makes three distinct cell types: neurons, oligodendrocytes and astrocytes. She uses microfluidic techniques for microspheres to deliver signals to the cells to tell them to differentiate into the desired cell types. For example, guggulsterone drives cells to become neurons, and purmorphamines result in motor neurons, a likely key to spinal cord injury repair. The result is mini spines for testing drugs or modelling created faster and with less waste than traditional techniques.
Dr. Iyer began her talk by saying “The spinal cord is not a monolith. We need to think about a regional specific approach to spinal cord injuries” adding that most differentiation protocols now fail to capture the regional diversity of the central nervous system. She noted that clinical trials in Parkinson’s and epilepsy have shown that regional specific therapies improve functional outcomes.
She said her goal is to develop translatable methods to differentiate cells at a manufacturing scale to bridge the gap between developmental biology and manufacturing. To systematically make any cell type, she is turning back to fundamental developmental biology. “First thing that happens in development is the cells figure out where they belong. Hox genes help them figure this out,” she said, so her lab looked at 39 Hox gene expression levels during development. Next cells need to figure out what to become. The lab looked at BMP and WNT signalling, which give rise to sensory neurons, and sonic hedgehog genes that give rise to motor neurons.
Iyer’s lab has been able to make region specific progenitors, ones that in normal development occur at specific times. She concluded that controlling temporal emergence of specific spinal cells will be important to integration into circuits, but said “We don’t know yet if this will be necessary for clinical repair. Most trials are using cells with a great deal of heterogeneity. A main focus of my lab is figure out if regional specificity will be necessary.”
A version of that first clinical trial did get restarted a couple of years after the initial shutdown. Asterias/BioTime, believing safety had been shown with the first five patients, moved the new trial to the upper cervical region of the spine where a relatively smaller repair can result in significant functional gain. In the trial, 21 of 22 patients treated gained at least one rung on the American Spinal Injury Association Impairment scale, with 32 per cent gaining two levels.
But the technology was hard to learn how to use and roll out. So the company, now renamed Lineage Cell Therapeutics, recently announced an amended protocol that uses a new platform to hold the needle for the delicate placement of the cells into the site of injury. Company CEO Brian Culley characterized the new device as easier to deploy and offering safer surgery. They also plan a wider patient population for the next trial. Until now all patients have been in the acute phase, usually within one month of injury. The new trial will enroll chronic patients, as much as a year or more out from injury.
Expanding the pool of patients also expands the pool of hope.
Don Gibbons
Latest posts by Don Gibbons (see all)
- Pluripotent stem cell clinical trials for SCI is the target of a “moonshot” consortium - May 28, 2024
- Hope vs hype and champions vs charlatans - August 22, 2023
- One pioneer’s dogged pursuit of personalized cell therapy - May 25, 2023






Comments