“Regenerative medicine news under the microscope” is a monthly feature highlighting a selection of impactful research findings and headlines across the many subfields of regenerative medicine.
Fall is finally here!
September really delivered – lots of interesting research to cover. In this edition, I discuss intestinal organoids re-imagined as a cell therapy, lab-created blood stem cells, regulatory T cells for tissue healing, and more.
Pick of the Month
Mending bowels with organoid soup
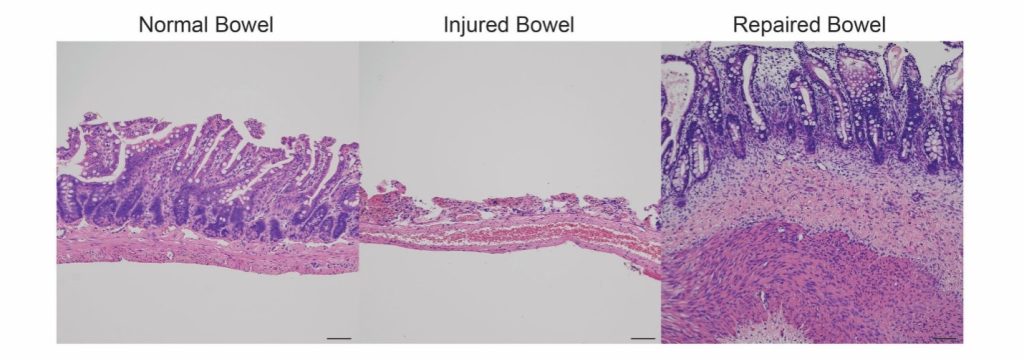
Imagine being able to repair injuries to the bowel across its many layers. This is the dream to better treat patients with inflammatory bowel diseases (IBDs), including Crohn’s disease and ulcerative colitis. Current treatments, such as steroids or biologics, suppress the patient’s immune system and may not always be effective; not everyone responds to the medications that are currently available, and some even stop responding to drugs that once worked for them. In serious cases, surgical resection of damaged tissue might ultimately be required.
This month’s Cell Stem Cell features promising work on this front, enlisting a locally delivered soup of intestinal organoids for the complex task of healing gut tissue. In Poling et al.’s protocol, intestinal organoids – originally developed by the same research group that conducted this study – were first generated from human cells, before being fragmented and introduced into the lumen of immunocompromised rodents with chemically and mechanically damaged bowels. Impressively, the cells remained in place to form healthy muscle, epithelium and blood vessels. Goblet cells (which produce mucin), paneth (antimicrobial) cells, and enteroendocrine (hormone-secreting) cells were also detected at the regenerated site. Importantly, there were no observed tumorigenic effects, and no off-target migration.
While the authors will be seeking FDA approval to investigate this new approach in a clinical trial, they aim to improve their manufacturing standards. They may also consider implementing a biological adhesive or hydrogel for better targeting, in addition to the potential adoption of organoids that incorporate neuronal components to better facilitate re-innervation.
While stem cells for IBDs are not necessarily new in theory, this is the first time that scientists are applying a stem cell product as complex as the organoid to do the job. For further reading, see the links below. Remember that this field is still in its early days, and that unregulated, private clinics claiming to offer “proven” treatments like these are not the solution. The only approval on the record is for Alofisel, a mesenchymal stem cell injection to treat complex perianal fistulas in Crohn’s patients – however, their Phase III clinical trial failed last year.
- Can Stem Cell Therapy Treat Crohn’s Disease? A great general overview of the topic by WebMD.
- Efficacy and safety of stem cell therapy for Crohn’s disease: a meta-analysis of randomized controlled trials. Qiu et al. in Stem Cell Research & Therapy.
- Clinical trial: Autologous Stem Cell Transplant for Crohn’s Disease and accompanying news piece from Mount Sinai reporting on one of their patients: Stem Cell Transplants Prove Effective for Patients With Severe Crohn’s Disease
Towards lab-created, transplantable blood stem cells
This one’s a world first that could potentially improve treatments for childhood leukemia and bone marrow failure. Ng et al. have developed human iPSC-derived hematopoietic stem cells that successfully engraft in animal models to become functional bone marrow capable of producing the entire “blood system” either in vivo or in vitro. Their results were similar to those from umbilical cord blood cell transplants, an established benchmark in the field.
The key to their success was a precisely timed growth factor exposure protocol involving the conversion of induced pluripotent stem cells (iPSCs) to blood vessels and then to blood cells. In addition, the authors found that they were able to achieve the scale, purity and preservation technique – freezing – required for clinical translation.
Alongside gene editing to correct any disease-causing mutations, the team envisions the application of this new technology in future blood stem cell and bone marrow transplants. Personalized strategies such as these not only address the issue of supply, but also greatly reduce the risk that the patient’s body will reject the cells.
The next stage, which would be a Phase I trial, is still about five years out according to the team. They’ll first have to address issues of consistency as both success rates and cellular diversity tended to vary across experiments, so these elements must be more tightly regulated before moving their protocol to the clinic.
For more on this breakthrough, check out James Woodford’s coverage at New Scientist. If the broader idea of blood derived from stem cells interests you, there was also a world-first clinical trial back in 2022 wherein red blood cells were generated from donor stem cells and infused into participating subjects. Dubbed the RESTORE trial, its mandate was different from the story above as it mainly focused on addressing the needs of individuals with sickle cell and rare blood types. Still, it’s really interesting work and worth keeping tabs on.
Giving stem cells new life: Transplant restores rare vision loss
Johanne Provost had not seen her family in three years – and I mean this in the literal sense. A rare autoimmune condition resulted in serious damage to the stem cells around her cornea, causing blood vessels and scar tissue to build up and block her vision. Monthly intravenous immunoglobulin treatments helped with the inflammation, but more would be required to restore her sight: She would need a stem cell transplant.
In similar cases, ocular stem cells could be acquired from the patient’s healthy eye to treat the diseased one. However, both of Ms. Provost’s eyes were affected by her condition. Given this new challenge, her ophthalmologist consulted Dr. Clara Chan, a surgeon and researcher in corneal diseases and therapies at the University Health Network (UHN). Dr. Chan’s team used stem cells from a deceased donor, giving them new life in Ms. Provost. UHN is the only institution in Canada that offers this particular procedure involving non-living donors, and Dr. Chan was the first doctor in Ontario to perform a deceased donor ocular limbal stem cell transplant.
Ms. Provost’s surgical procedure was a success. Following her recovery, she was able to see her family again. This type of treatment isn’t necessarily new, but it’s nice to be reminded of regenerative medicine success stories and the actual impact they have on people’s lives.
An immune cell for tissue healing
Regulatory T cells (Tregs) are best known for their control over the immune response, preventing autoimmune diseases and other pathological reactivity. Work published in Nature Communications this month adds to this repertoire of utility, demonstrating that these cells can also improve healing in multiple tissues including skin, muscle and bone.
Once applied to the injured site, Tregs detect and convert to the appropriate cell type required by the damaged environment, upregulating gene expression associated with both healing and immunomodulation. They also flood the environment with beneficial factors from other healing cell types, and reduce the piling on of toxic immune cells that might adversely affect repair.
Interestingly, it’s already been found that these cells do naturally accumulate at injury sites; however, the trick here is augmenting the numbers and bringing them in as early as possible to maximize their benefit. Importantly, these cells don’t have to share the genetics of the patient, positioning them well as a potential off-the-shelf solution.
This work involved both rodent and human Tregs tested in mice, so of course these findings must be confirmed in clinical trials.
Additional recommendations
I’ve decided to change up the format of this section – listing journal names might introduce some degree of bias here, so I’ll be listing paper titles only from now on.
- Transplantation of chemically induced pluripotent stem-cell-derived islets under abdominal anterior rectus sheath in a type 1 diabetes patient.
- The field seems cautiously optimistic about this work, which has made global headlines this month. It’s really positive that we’re moving towards finding ways to avoid lifelong immunosuppression via iPSCs or encapsulated solutions (as with Vertex’s newer VX-264), although this patient was already on immunosuppressants for a different reason. Also, diabetes is technically an autoimmune condition, so it remains to be seen how long these islets will last. A balanced and thorough news piece on this can be found at Nature.
- These two pieces go well together: Doctors cured her sickle-cell disease. So why is she still in pain? And Sickle cell gene therapies roll out slowly.
- Hearing directly from patients about their experiences with these new treatment options is always critical.
- Therapeutic efficacy of intracerebral hematopoietic stem cell gene therapy in an Alzheimer’s disease mouse model.
- Kyoto University Hospital seeks to treat Type 1 diabetes using iPS cells.
- Flint woman with leukemia may be 1st to get stem cell transplant from deceased donor.
- DNA methylation controls stemness of astrocytes in health and ischaemia.
- Development of adeno-associated viral vectors targeting cardiac fibroblasts for efficient in vivo cardiac reprogramming.
- Graft survival of major histocompatibility complex deficient stem cell-derived retinal cells.
- Orthocell’s collagen-membrane dental-regeneration product Striate+ debuts in Canada.
- Organoid culture promotes dedifferentiation of mouse myoblasts into stem cells capable of complete muscle regeneration.
- Spatial transcriptomics defines injury specific microenvironments and cellular interactions in kidney regeneration and disease.
- TEX264 drives selective autophagy of DNA lesions to promote DNA repair and cell survival.
- p65 signaling dynamics drive the developmental progression of hematopoietic stem and progenitor cells through cell cycle regulation.
- Long-term engrafting multilineage hematopoietic cells differentiated from human induced pluripotent stem cells.
- Multifunctional injectable microspheres for osteoarthritis therapy via spatiotemporally modulating macrophage polarization and inflammation.
- rADSC-loaded tubular units composed of multilayer electrospun membranes promoted bone regeneration of critical-sized skull defects.
- Efficient decellularization of human fetal kidneys through optimized SDS exposure.
- Mesenchymal stem cell cryopreservation with cavitation-mediated trehalose treatment.
- Effect of photobiomodulation therapy with 660 and 980 nm diode lasers on differentiation of periodontal ligament mesenchymal stem cells.
- Startup from George Church’s lab raises $75M to develop ‘supercell’ medicines.
- Regenity Biosciences Receives Regulatory Approval for Collagen Dental Membrane in China After First-of-its-Kind Breakthrough Clinical Study.
- RION Announces Initiation of Phase 1b Clinical Study to Evaluate Purified Exosome Product™ (PEP™) for Knee Osteoarthritis.
- Tempo Therapeutics Announces First Patient Dosed in Clinical Trial of TT101 For Tissue Repair in Skin Cancer Surgery.
- A longevity-specific bank of induced pluripotent stem cells from centenarians and their offspring.
- Individual variation in the emergence of anterior-to-posterior neural fates from human pluripotent stem cells.
- A mathematical insight to control the disease psoriasis using mesenchymal stem cell transplantation with a biologic inhibitor.
- Comparison of methods for cancer stem cell detection in prognosis of early stages NSCLC.
- Septo-dentate gyrus cholinergic circuits modulate function and morphogenesis of adult neural stem cells through granule cell intermediaries.
- Inflammation-induced epigenetic imprinting regulates intestinal stem cells.
- RHEACELL announces FDA approval for Phase 3 study in refractory, non-curable CVU.
- Spaceflight-induced contractile and mitochondrial dysfunction in an automated heart-on-a-chip platform.
- Insidious chromatin change with a propensity to exhaust intestinal stem cells during aging.
- Robust differentiation of human pluripotent stem cells into mural progenitor cells via transient activation of NKX3.1.
- Nephrotic syndrome and portal hypertensive ascites after allogeneic hematopoietic stem cell transplantation: a rare manifestation of chronic graft-versus-host disease.
- A living organoid biobank of patients with Crohn’s disease reveals molecular subtypes for personalized therapeutics.
Noted RMAT Designations
- Poseida Therapeutics Receives Regenerative Medicine Advanced Therapy (RMAT) Designation from FDA for P-BCMA-ALLO1 to Treat Relapsed/Refractory Multiple Myeloma.
- BridgeBio Receives FDA’s Regenerative Medicine Advanced Therapy (RMAT) Designation for BBP-812 Canavan Disease Gene Therapy Program.
- Obsidian Therapeutics Receives FDA Regenerative Medicine Advanced Therapy (RMAT) Designation for OBX-115 for the Treatment of Advanced Melanoma.






Comments