CRISPR-Cas9 is not only a revolutionary technology, it is also a topic that never ceases to intrigue. From gene editing to agriculture, the possibilities are seemingly endless. In medicine, CRISPR-Cas9 has shown promising results in curing genetic blood disorders and developing new therapies for cancer. However, concerns about off-target editing and delivery remain significant. Fortunately, researchers are working tirelessly to tackle these limitations and improve the technology. In this blog, I will dive deeper into the latest advancements in CRISPR-Cas9 technologies, including Prime Editing (PE), CRISPR-associated transposases (CASTs), and molecular syringes. These breakthroughs represent a significant step forward in the ability to modify the genome with unprecedented precision, enabling scientists to develop new therapies for a wide range of diseases. So, let’s take a closer look!
A CRISPR-Cas9 Primer
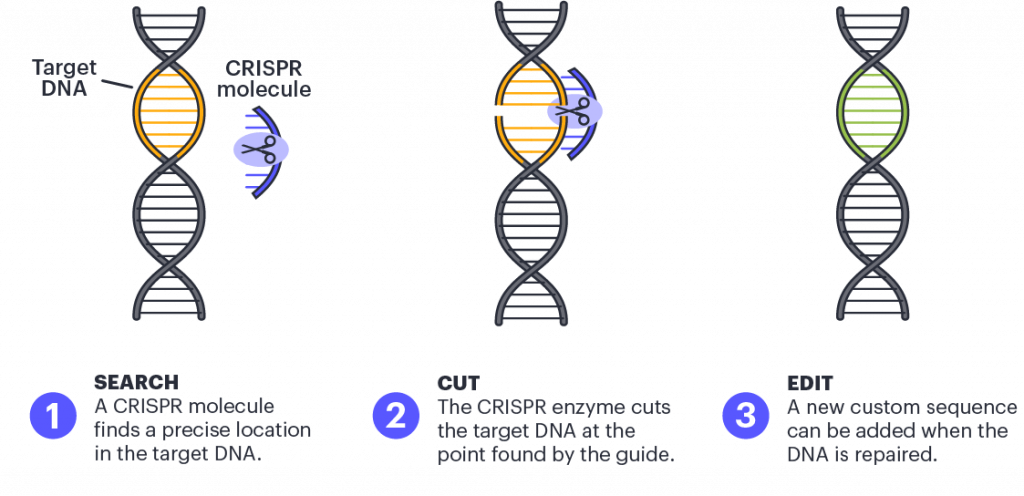
Before we delve in, we have not discussed CRISPR-Cas9 on a molecular level here at Signals for a while (read other posts here), so I’m providing a brief overview. The system is based on the CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) mechanism, which was originally discovered as a bacterial immune system that defends against viruses and other foreign DNA. At its core, the system consists of two key components: the CRISPR RNA, which acts as a guide to a specific DNA sequence, and the Cas9 protein, which acts as the molecular scissors to cut double-stranded DNA. The cell’s natural DNA repair mechanisms can then be used to either repair the cut site or introduce new DNA sequences to replace the cut section.
The latest CRISPR-Cas9 advancements from the world of biomedical research
Precisely using CRISPR-Cas9: Prime Editing
In response to concerns about CRISPR-Cas9 fidelity, researchers have implemented Prime Editing (PE), a focused approach that can edit single DNA bases. In PE, the Cas9 molecular scissors are transformed into a nickase (nCas9), which is like a surgical scalpel that cuts only one DNA strand, avoiding the messy double-stranded breaks that can introduce errors.
However, a new study by Lee et al. has raised concerns about existing nCas9 nickases, which were found to produce off-target double-stranded breaks. Using structural and protein engineering approaches, they uncovered that nCas9 had higher activity than expected, necessitating further modification. Utilizing the newly developed nCas9* for PE, the authors achieved true nickase capabilities. This represents a breakthrough in the field of gene editing, providing scientists with an even more precise toolkit to selectively modify the genome with minimal side effects. With PE already being used to treat diseases like sickle cell anemia, the improved accuracy of nCas9* will only accelerate efforts to develop highly targeted therapeutic gene therapies.
Enabling copy and paste within the genome: CRISPR-associated transposases (CASTs)
Programmable CRISPR-Cas9 editing enabled by approaches like PE has improved the fidelity of genetic modifications but is limited to 50 nucleotides of editing. While attractive for single nucleotide genetic defects, this limits applicability for diseases like G6PD deficiency or Huntington’s. Having the ability to remove faulty genes and insert chunks of DNA as large as 50,000 nucleotides would open new avenues for gene editing therapeutics.
A team from Columbia University has made significant progress by adapting bacterial CRISPR-associated transposases (CASTs) for inserting large chunks of DNA in a single step within human cells. Transposases are proteins that move large DNA chunks in human cells, often without specificity. Building off previous efforts, Lampe et al. utilized CASTs from Vibrio cholerae to enable precise genome insertion of large pieces of DNA. The team elegantly highlights the high-fidelity DNA insertion of up to 5000 nucleotides, given that this tool avoids the use of double-stranded breaks (DSBs), like PE. Additionally, as existing tools to insert large DNA chunks relied on complex multistep pathways, their use in therapies was limited. The development of CASTs represents an exciting path forward, coupling the specificity of PE with the gene-editing ability of transposons. However, the authors caution readers toward the limitation of their efforts. Unlike the other tools presented in this blog, the study of CASTs was based on in vitro work and requires further in vivo studies. Nonetheless, this advance represents a significant step forward in the ability to modify large segments of the genome.
Delivery of the CRISPR-Cas9 System: Molecular syringes
Developing innovative tools is one aspect of CRISPR-Cas9 research. However, the tools’ utility fundamentally depends on their delivery to the regions of the body that need repair. This is a two-fold barrier, as the tools need to be delivered among 200 cell types and then pass through the cellular membrane. The solution to this problem may lie in a common foe: bacteria.
Researchers from the Zhang group have developed a customizable toolkit to introduce protein cargo into human cells by borrowing a bacterial injection system known as Photorhabdus Virulence Cassettes (PVCs). In nature, PVCs deliver toxic protein cargo to kill insect cells. Kreitz et al. instead focused on sensitizing these PVCs toward human cells by adding adaptors that recognize membrane receptors. When they packaged these syringes with a toxic protein cargo, they saw specific cell death. In contrast, when they loaded PVCs with the proteins in the CRISPR-Cas9 system, they noted specific gene editing.
Building on this in vitro work, the researchers also injected PVCs containing fluorescent cargo into mice and visualized this signal near the injection site. What’s exciting about these findings is the customizable nature of this technology. Through simple manipulations, PVCs can be loaded with specific cargo and targeted to specific cell types. This could involve targeting CRISPR-Cas9 to edit specific cell types or delivering targeted therapies to tumour cells. Importantly, the Kreitz et al. in vivo work demonstrates potential gene therapy applications given the specific delivery . These combined efforts represent an important step in adapting molecular tools to deliver protein cargo for both in vitro and in vivo applications, opening the door for further development of PVCs in these applications.
RNA-lipid nanoparticles: a breath of fresh air
Despite the current advances from groups like Kreitz et al., lung diseases are often the most difficult to deliver protein cargo to, given the effort to protect the lungs from foreign particles. This necessitates specialized delivery tools for any treatments targeting lung diseases, like cystic fibrosis.
With the success of the mRNA-based COVID-19 vaccinations, Li et al. took advantage of this momentum to create RNA-lipid nanoparticles specifically targeting mouse lung tissue, avoiding traditional clearance issues. In particular, Li et al. developed a method for delivering CRISPR-Cas9 therapy to the lungs using lipid nanoparticles. They identified an efficient lipid, called RCB-4-8, which can target both ciliated and club epithelial cells in the lung, enabling gene editing. They also developed a dual-delivery approach to limit off-tissue targeting editing of these CRISPR RNA-lipid nanoparticles. Future studies will explore the performance of these CRISPR RNA-lipid nanoparticles in diseased animal models. Another avenue the researchers are exploring is ways to improve aerosolization, enabling the ability to inhale future therapeutics.
The latest advancements in CRISPR-Cas9 technologies, such as Prime Editing, CRISPR-associated transposases and novel molecular syringes, represent significant breakthroughs in the field of gene editing. These innovations bring us closer to developing more effective therapies for a wide range of genetic disorders, including those previously considered incurable. Most excitingly, and a tad on the side of burying the lede, these developments were all recently published in 2023, meaning the best is yet to come. As a scientist who uses CRISPR-Cas9 for tool development in the basic sciences, it is a privilege to read about the creative laboratories on the cutting edge (no pun intended) of gene therapy developments. In the meantime, as researchers continue their tireless work, I remain optimistic that CRISPR-Cas9 will bring even more exciting possibilities for genetic engineering and medicine, and I hope you do too.







Comments