We’ve spent a lot of time swooning over CAR T-cells and CRISPR here at Signals, but recently a potential up-and-coming leading lady in the gene and cancer therapy fields has caught our eye.
Our interest was piqued by a recent press release from The Max Delbruck Center for Molecular Medicine (MDC) announcing the continuation of a formal partnership with PlasmidFactory to optimize their Sleeping Beauty system to advance gene-therapy cancer treatment. MDC plans to use its “Sleeping Beauty 100x” system to manipulate genes for cancer treatments and, with PlasmidFactory’s help, aims to make their system safe and scalable for human use.
Our new love interest, Sleeping Beauty 100x, is a transposon (“jumping gene”): a DNA sequence that can move, or “jump,” within a genome. Transposons are an important and necessary part of evolution as they allow for the creation (and/or reversal) of random genetic mutations within and between chromosomes. A transposon system has two main components: the transposon DNA sequence and an associated transposase (enzyme). Transposon DNA sequences, which can contain genes, are flanked by specific repetitive DNA sequences called inverted repeats (IRs). The IRs are recognized by transposases, which cut the DNA, leaving a “sticky end” at the cleavage site. These “sticky ends” are unique, and can only bind to specific complementary parts of DNA elsewhere in the genome, ensuring insertion specificity. DNA replication/repair machinery connects the transposed sequence to the native DNA strand.
The original Sleeping Beauty (SB10) transposon was synthesized in 1997, after being awoken from her long evolutionary sleep (she was reconstructed from fossil transposase sequences). The SB10 system has been used extensively to knockout or insert genes, generating animal models and cell lines to test for gene function. After her debut, our girl-next-door received an extensive make-over, upgrading her transposase to be 100 times more efficient in gene transfer – a transformation so exciting that she was namedf “Molecule of the Year” in 2009 by an international body. Oh, and she goes by “SB100x” now.
The SB100x system is an attractive option for gene transfer as it avoids a host cell’s defenses against viruses, which are among the more common vectors for gene transfer presently. The SB100x system is also (theoretically) cheaper than upscaling a viral transduction system. It is also more efficient and precise than passive non-viral DNA transfer. I found this review article, which covers the benefits and drawbacks of Sleeping Beauty transposons, helpful.
As alluded to in MDC’s press release, the group plans to use their SB100x system to engineer CAR T-cells. If you check out my previous post on CAR T-cells, you’ll notice that the “CAR” (chimeric antigen receptor, a hybrid of regular T-cell machinery and external receptor to a target of interest) is constructed in a test tube, then a viral vector is used to insert the chimeric DNA into the T-cells. MDC and PlasmidFactory propose to perfect a plasmid (circular DNA) delivery system for the SB100x transposase and transposon containing the chimeric gene of interest, which can then be introduced directly into a patient’s T-cells. They have goals of using CAR T-cells not only as cellular cancer therapies, but also as ways to treat autoimmune diseases, like anti-NMDA receptor encephalitis.
Some of you might be screaming “but you already have CRISPR (see my previous post), how could you turn your back on her!?” Agreed. The CRISPR-Cas9 and SB100x systems are fairly similar. Both are tools that are used to cut DNA, removing or adding in new sequences. In the context of CAR T-cell generation, both technologies negate the use of a virus to insert the CAR DNA. There are some subtle differences (see Figure), that may make one system preferable in a particular scenario.
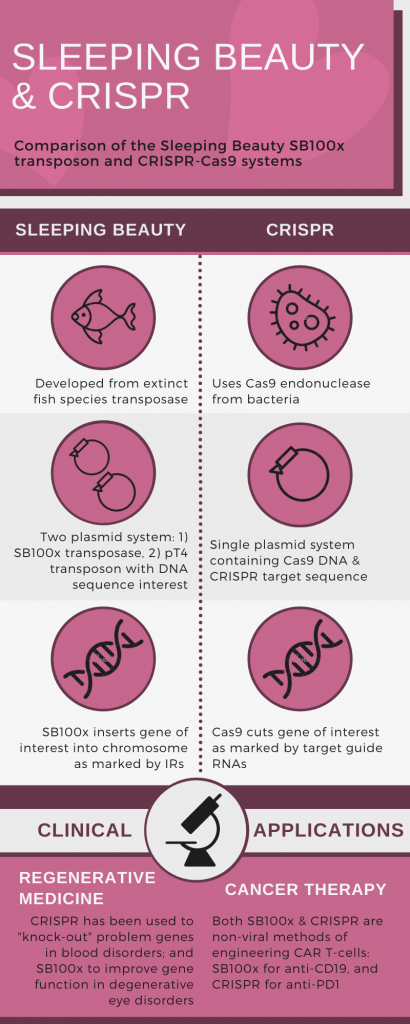 For example, the original role of CRISPR-Cas9 was for bacteria to cut foreign bacteriophage DNA, rendering it inert; whereas, the Sleeping Beauty transposon cuts and pastes pieces of DNA into new places. It is possible, with further use in genetic engineering, CRISPR-Cas9 may be found to be better suited to “knocking out” targets, and SB100x for restoring or creating new function.
For example, the original role of CRISPR-Cas9 was for bacteria to cut foreign bacteriophage DNA, rendering it inert; whereas, the Sleeping Beauty transposon cuts and pastes pieces of DNA into new places. It is possible, with further use in genetic engineering, CRISPR-Cas9 may be found to be better suited to “knocking out” targets, and SB100x for restoring or creating new function.
Both genetic engineering technologies are exciting and worthy of our adoring attention. Our “Princess SB100x” has been quietly making great strides, with successes in pre-clinical in vitro and ex vivo models of age-related macular degeneration, and also in engineering anti-CD19 CAR T-cells. CRISPR has shown the beginnings of success in treating genetic hematological disorders and cancer in early clinical trials. Perhaps a fairy tale ending for cancer therapies and regenerative medicine isn’t so far away….
Sara M. Nolte
Latest posts by Sara M. Nolte (see all)
- Ottawa researchers make tumour-marker negative cancers positive with oncolytic viruses - December 19, 2024
- Cell-based therapy approved as alternative to standard UCB transplant for hematological malignancies - June 21, 2023
- Finding the perfect match: breaking down the science of organ and stem cell transplant matching - April 18, 2023







Comments