We heard it first.
If you attended the virtual Vancouver version of the 2020 Till & McCulloch Meetings, you had the opportunity to hear from Dr. Janet Rossant, Chair of the Expert Panel on the Approval and Use of Somatic Gene Therapies in Canada, giving us a preview of the “From Research to Reality” report just issued this week by the Council of Canadian Academies (CCA). Her talk was part of a session on Gene Editing: Policy and Society, chaired by this year’s Till & McCulloch Award winner Dr. Bartha Knoppers.
The CCA is a not-for-profit organization that brings together experts to provide guidance and inform decision-making on complex (sometimes controversial) scientific topics that affect the public. Dr. Rossant’s talk, based on the CCA report, was titled “Access and affordability challenges with somatic gene and engineered cell therapies.” The other panel members were William Charnetski, Ubaka Ogbogu and Dr. Harold Atkins.
Dr. Rossant explained that access and affordability challenges are being discussed at this time because Health Canada has now approved four gene therapies (Kymriah, Yescarta, Spinraza and Luxturna) and there are more in the pipeline. In fact, based on the numbers of clinical trials in progress, we could see 10-20 gene therapy products approved every year for the next few years. This is great news for patients, but only if we can tackle cost and accessibility issues.
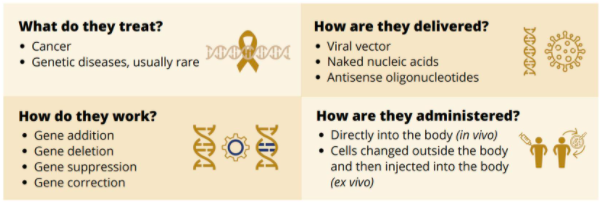
So what is a somatic gene therapy? Dr. Rossant describes it as follows: “the introduction, removal, or change in genetic material – specifically DNA or RNA – into the cells of a patient to treat a specific disease.” For example, in CAR-T cell therapy, a patient’s own immune system cells are removed from the blood, modified in a lab to intensify their natural inclination to attack foreign (cancer) cells and then inserted back into the patient. In the case of Luxturna, a treatment for retinal disease, a normal copy of the RPE65 gene is inserted directly into a patient’s retinal cells and the gene instructs the cells to produce the protein that converts light to an electrical signal in the retina to restore vision loss.
Because the therapies treat different diseases, are delivered differently, work differently and get administered differently, the regulatory environment must be flexible.
(Screen shot from presentation by Dr. Janet Rossant, Till & McCulloch Meetings 2020)
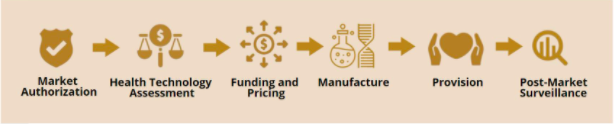
In Canada, there are six stages of approval and use for a new therapy.
(Screen shot from presentation by Dr. Janet Rossant, Till & McCulloch Meetings 2020)
You obtain market authorization from Health Canada, based on positive clinical trial results. CADTH and INESSS (Quebec) conduct the health technology assessment in parallel and look at whether the new drug is cost effective and better than other products on the market.
Funding and pricing are complicated issues because of the novelty of gene therapies and the fact long-term safety and efficacy are unknown. Gene therapies also come with a very high price tag (most are one time payments for a permanent cure rather than treatments that continue through a patient’s lifetime). For more background on this topic, I recommend this post on reimbursement from CCRM’s former “regulatory guy” (his words).
Keep in mind that another hurdle we face in Canada is the fact reimbursement decisions are left to each province. At this time, governments, therapy developers and insurance companies are exploring different reimbursement solutions, from subscription models, to pay-for-performance, to patient assistance programs and lotteries – all are being contemplated (and more!). Down the road, post-market surveillance will be another means of informing regulatory and pricing of approved gene therapies as more evidence is available for analysis.
Dr. Rossant concluded her talk by reminding us that these challenges are being experienced around the world, but Canada has some particular nuances due to federal and provincial jurisdictional issues. However, we also benefit by the excellence of our research and I would add our collaborative nature. As Dr. Rossant stated, we have the opportunity to position Canada as a leader in gene therapies by building a supportive landscape.
Stacey Johnson
Latest posts by Stacey Johnson (see all)
- Right Turn: Top 10 blogs from 2025 - January 9, 2026
- Right Turn: Season’s greetings and upcoming event - December 25, 2025
- Right Turn: Stem cell supplements: A growing market with growing risks - December 19, 2025







Comments