The Alliance for Regenerative Medicine (ARM) delivered its State of the Industry Briefing (#CGSOTI23) at Biotech Showcase, held January 9–11, 2023, in San Francisco, California.
Tim Hunt, CEO, of the Alliance for Regenerative Medicine (ARM) kicked things off by outlining ARM’s role in convening the regenerative medicine (RM) sector. He spoke of ARM’s efforts to build a robust RM and cell and gene therapy community by providing data and analysis, and revealed that as of the latest count ARM has over 475 members globally.
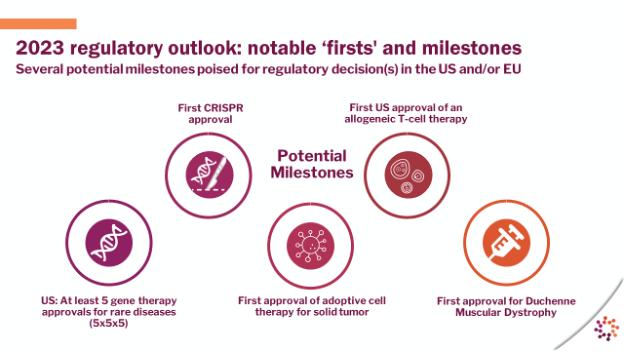
Throughout the presentation, there was a strong emphasis on the industry’s acceleration, driven by scientific advances that are bringing meaningful developments for patients.
“If you go back, 20 years, 25 years, there were years where the progress for patients seemed like a crawl. It was a long hard slog for some of the prominent academics that have pioneered amazing technology, and the companies that are out there trying to translate the science into clinical advances, it was a crawl,” says Mr. Hunt. “Then about five years ago, we began to walk. We started to see more and more approvals and meaningful developments, and I would argue that, in 2022, we started to jog. We started to make meaningful progress for patients.”
Noting that it took over five years to get the first five approvals in the United States for gene therapies for rare diseases, Mr. Hunt says that the outlook for the year ahead could double that figure in a single year, heralding a further five approvals.
Source: Alliance for Regenerative Medicine State of the Industry Briefing
The prevailing theme of the presentation was that although the science behind cell and gene therapies is advancing rapidly, the readiness of health care systems to adopt them is not keeping pace.
With more therapies addressing indications with large potential patient groups who could benefit from them, the eligible patient population is set to grow significantly. This, says ARM, could create a “forcing function” that creates the necessary impetus for payers to adapt. 2023 and beyond are likely to be the biggest test yet for payers, and the question on ARM’s mind is can they modernize to keep pace with the science?
The presentation recognized progress in the United States – reserving its sterner words of warning for the European Union – acknowledging that the FDA is evolving to meet regulatory demands. However, ARM says that questions still linger about payer readiness and that modernization is needed across public and private payers to expedite access.
Legend:
- OTAT: Office of Tissues and Advanced Therapies
- OTP: Office of Therapeutic Products
- PDUFA VII: Prescription Drug User Fee Act, seventh iteration
- CMC: Chemistry, Manufacturing and Control guidance
The EU on the other hand was described as “a flashing yellow light of caution for patient access” in the State of the Industry presentation and a subsequent ARM tweet, which suggested the bloc could be losing its RM leadership status.
Source: Alliance for Regenerative Medicine State of the Industry Briefing
“I was pleased to attend a briefing we did in November in Brussels at the European Parliament, and I think it’s helpful to speak truth to friends,” said Mr. Cook, “and so when I was over there and gave some remarks I said, listen, Europe clearly has been a leader in cell and gene therapy going back decades. A lot of major innovations, top medical centres, the talented workforce etc., and at the same time, we see the European Union as a flashing yellow light of caution for patients. There have been roadblocks in reimbursement that I think we’re all familiar with. That’s complicated patient access. Seven of the 24 [available] ATMPs have been withdrawn from the market in Europe primarily for economic reasons, and clinical trials and investment are not ideally where we would like to see them. It’s been stagnating, and there are only three new phase-one clinical trials that we saw in 2022. That’s the rough news. The good news is there’s another choice. There’s a fork in the road. And so, we think that there is a better path in Europe.”
ARM says that the European bloc now has a once-in-a-generation chance to reverse this trend and is calling on European leaders to revise the EU Pharma Legislation via the EU Health Technology Assessment.
In closing, Mr. Cook summarized that we, as an industry, took our pace up to a jog in 2022, that the year ahead is likely to be a seminal one, and though hurdles exist, by overcoming them real running is attainable. Watch the full industry update presentation on the ARM YouTube channel.
My next ecosystem overview blog, coming in February, will look at how the California Institute for Regenerative Medicine has been strengthening the U.S. ecosystem, with key updates from south of the border that you may have missed.
Cal Strode
Latest posts by Cal Strode (see all)
- What’s in the mix for 2026: ARM’s State of the Industry briefing - January 28, 2026
- World AIDS Day: Update on HIV cure research and gene therapies - December 1, 2025
- Headwinds and tailwinds for cell and gene therapy under the second Trump administration - March 11, 2025








Comments