In current organoid technologies, human pluripotent stem cells are guided to differentiate into 3D structures that resemble the corresponding human in-vivo organ, which allows researchers the opportunity to study the organ’s specific cells and function. However, organoids are limited by their inability to mimic circuits or interactions between cells of different organs. Building on their 2017 seminal work that introduced the concept of assembloids (where multiple organoids are integrated together), Dr. Sergiu Paşca and colleagues at Stanford University study the complex interactions that govern neural development and function.
In a recent preprint published March 2024, the team created an assembloid to model one of the major pain circuits of the ascending sensory neurological pathway so that they might be able to investigate the mechanisms of pain in a more holistic way. For the sake of understanding their model in their research, let’s say you get a burn on the right side of the body (noxious stimulus). The neurons at the site of injury carry the signal to the ipsilateral side (same side of injury) of the dorsal horn of the spinal column. Once in the dorsal horn, these neurons will release certain substances onto the post-synaptic neurons of the spinothalamic tract. From the dorsal horn, the spinothalamic tract crosses sides of the spinal cord and ascends to the thalamus (relay station of the brain) synapsing to post-synaptic neurons that then relay the pain to the somatosensory cortex. Here is where the perception of pain is experienced.
It is important to note that while this is one of the ascending neural pathways that process pain signals, it’s not the only pathway necessary to “feel” pain, and it’s also not necessarily sufficient on its own to feel pain – other circuits are needed. However, the research team’s current assembloid model for this particular pain pathway could be a great starting point to investigate alternatives to opioid pain medication, pain sensitivity and insensitivity, and the complex nature of the human perception of pain in general (which will be discussed in more detail below).
Opioid crisis
In the 1980s and ‘90s, pharmaceutical companies began to aggressively market synthetic opioid painkillers while downplaying their addictive potential. The addictive potential (due to dopamine level changes and enhanced mood effects) as well as the other physiological effects opioids have on the body lead to tolerance, dependence and withdrawal symptoms, which make people sick when trying to recover from the addiction. If someone trying to recover from addiction goes back to using opioids later, they can be at high risk for an overdose. What would have been a standard dose while their tolerance was high can later be lethal and cause accidental death.
We are in what many call an opioid crisis in North America due to physician over-prescribing and an increase in the illegal use and selling of opioids.
In the United States, in 2016, in the rush to “fix” the opioid crisis, the Centers for Disease Control and Prevention (CDC) advised physicians and pharmacists to harshly limit opioid prescriptions. However, the CDC later recognized their initial reformed guidelines as perhaps too extreme and updated them. However, many doctors in the United States continue to worry about prescribing the painkiller medication and, as a result, many chronic pain patients were (and sometimes still are) left chronically debilitated without the medication. Although opioids can be addictive, and some patients do develop a dependence on the drug, most patients use it with the single purpose of mitigating pain. These patients do not consider themselves addicts, and it might be the only way (currently) for patients to manage their persistent, chronic pain and to have some quality of life.
Bench to bedside translational challenges
Researchers and physicians are currently frustrated with the lack of translational progress in the pain field, because huge gains in basic science knowledge obtained using animal models have not led to the development of new clinically effective alternative drugs. Challenges in bench to bedside translation have a lot to do with differences between animals and humans. Animal models of pain don’t reflect the complex intermingling between hypersensitivity levels in people; the different dimensions of pain, including nociceptive – from peripheral tissue injury or extreme temperature; inflammatory, neuropathic (nerve damage), and idiopathic (chronic pain that doesn’t involve nerve damage or inflammation); the underlying cause of each; the influence on sex, genes and social factors (i.e. loneliness) that contribute to pain perception; and, various behavioural/movement responses people experience in relation to pain.
The assembloid to model pain
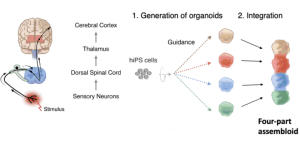
As mentioned, Dr. Pașca and his team at Stanford have recently taken the organoid a step further by physically and functionally integrating multiple separately engineered organoids together into what he coined as an assembloid to study the pain circuit described earlier. For their pain circuit assembloid, their preprint explains their generation of the somatosensory organoid (sensory neurons that would be located at the skin), dorsal spinal cord organoid, diencephalic organoid (this constitutes the thalamus relay station), and cerebral cortical organoid (constituting the neurons of somatosensory cortex) from human induced pluripotent stem cells, and their subsequent integration.
They used both RNA sequencing and protein expression analysis to verify they achieved the right cell types corresponding to the in-vivo ascending somatosensory pathway for each organoid. They also verified that neurons from each organoid (sensory neurons, spinal cord neurons, thalamus, somatosensory cortex) were both structurally and functionally connected through neuronal synapses in the pathway sequence and that the formation of these connections was capable of synchronized activity across the pathway in response to somatosensory-like stimuli. Finally, they were able to use this assembloid to investigate whether they can start to recapitulate the sensory dysfunction that leads to disorders involving the experience of pain insensitivity, thereby unravelling the pathophysiology of pain-related disorders.
Despite the need for further refinement, optimization, and study of their model, they hope that it can be used to unravel the process behind not only pain insensitivity but also disorders that involve heightened perceptions of pain, and even psychiatric disorders. Importantly, their assembloid can also capture human-specific aspects of the sensory system, which mitigates the challenge of translating animal studies to unique human physiology and experience. One major caveat mentioned by Dr. Pașca previously is that because the human brain is shaped by sensory experiences, which it is exposed to during development, and because lab-grown tissues do not have access to these cues, assembloids cannot fully mimic real human tissues or experiences.
Nonetheless, other research labs have used assembloids to investigate other conditions/diseases. The Pașca lab has also developed other induced pluripotent stem cell-derived assembloid models, such as the brain-to-skeletal muscle motor circuit, in the hopes of studying disorders involving movement and motor neurons (i.e. ALS). Despite limitations, the assembloid model holds exciting promise in closing the gap between testing and screening of therapeutic approaches and clinical translation for pain.






Comments