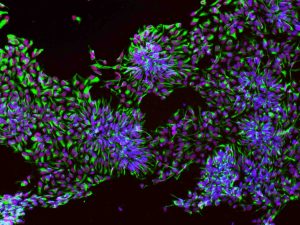
“The Brain Stem Cells” by Malvin Jefri. The neural progenitor cells (NPCs), stained with DAPI (blue), Sox1 (red), and nestin (green), can differentiate into neurons that inhabit our heads and give us our intelligence. The purity of NPC population in a given culture is the key to successful investigation of neurological disorders. Submission to 2019 Cells I See contest: https://cellsisee.ccrm.ca/2014-cells-i-see/
Imagine slowly losing control of your body and the muscles necessary to live healthily, until you eventually lose your ability to breathe. This disease is known as Amyotrophic lateral sclerosis, or ALS, and is caused by the degeneration of motor neurons that send signals for muscle movement, leading to muscle wasting and eventual death.
Every year, about 1,000 people in Canada and 5,000 people in the U.S. are diagnosed with ALS, according to national statistics. ALS occurs in approximately two people for every 100,000 individuals across the world, with a prevalence of up to 7.4/100,000. It is the most common motor neuron disease and life expectancy following a diagnosis is usually about 2 to 5 years.
ALS is a neurodegenerative disease of the nervous system that affects nerve cells in the brain and spinal cord. It mainly involves upper motor neurons (UMN) and lower motor neurons (LMN), manifested as a progressive paralysis. Nervous system symptoms include spasm, muscle stiffness, hyperreflexia, and pathological reflexes, which are signs of UMN involvement; muscle weakness and atrophy are signs of LMN injury. ALS usually progresses rapidly, with patients dying of respiratory failure after the onset of the disease.
Originally called Charcot’s triad, ALS was identified by a French neurologist, Jean-Martin Charcot in 1869. It became known as Lou Gehrig’s disease after gaining international popularity, when the New York Yankees’ greatest baseball hitter, Henry Louis Gehrig, was diagnosed with the disease in 1939.
The pathogenesis of ALS involves a complex interplay of genetic and environmental factors. Although it is still unclear, mutations in several genes have been identified to play a crucial role in the development of ALS. The genes involved in ALS affect various cellular processes, such as protein degradation, cytoskeletal dynamics, and immune response.
The accumulation of misfolded proteins are some of the key mechanisms implicated in ALS. Additionally, neuroinflammation and immune system dysregulation play a role in ALS progression. Activated microglia and astrocytes, the immune cells of the central nervous system, release inflammatory molecules that contribute to motor neuron damage. Furthermore, dysfunction in the blood-brain barrier allows immune cells from the periphery to infiltrate the central nervous system, exacerbating the inflammatory response. These alterations ultimately lead to motor neuron degeneration, axonal loss, and muscle weakness, which are characteristic features of ALS.
How can you cure ALS?
Despite the fact that the understanding of the molecular causes of ALS have advanced rapidly in recent years, these findings have not yet resulted in a cure or a reversal of the disease. Instead, case-based supportive drug therapies and occasional intensive care treatments are being applied. In addition, symptomatic therapies play an important role in improving the quality of life, while slowing its progression and reducing discomfort.
Emerging research suggests that there is potential for stem cell therapy and regenerative medicine as a cutting-edge treatment for ALS.
Stem cell therapy for ALS
The use of stem cells to treat ALS by replacing lost motor neurons is a promising potential treatment that began in the 1990s. Possessing the ability to self-renew and differentiate into any cell type, they were initially proposed with the aim of ameliorating the deterioration of the muscle by replacing and repopulating lost or damaged muscle neurons for transplant into ALS patients. However, it proved difficult to achieve in practice due to the immune system’s response to transplanted cells, local factors and a diseased environment. The focus, while still on stem cells, has been shifted towards a supportive approach where transplanted stem cells provide a nurturing, neuroprotective microenvironment by secreting neurotrophic factors and differentiating into healthy, non-neuronal or modulatory cells, such as astrocytes and microglia, that synapse with diseased muscle neurons.
Since then, various types of stem cells have been investigated for their therapeutic potential in ALS, each possessing distinct characteristics and advantages. Some of them include human embryonic stem cells, fetal neural progenitor cells, induced pluripotent stem cells and mesenchymal stem cells.
Human embryonic stem cells
Human embryonic stem cells (hESCs) are derived from the inner cell mass of blastocysts and exhibit self-renewal and differentiation capabilities into various cell types. Notably, studies have demonstrated the differentiation of hESCs into motor neuron-like cells expressing specific markers relevant to ALS. Preclinical investigations involving the transplantation of motor neuron progenitors (MNPs) derived from hESCs into ALS animal models have shown promising results, including survival, differentiation, and neurotrophic support that can reduce motor neuron loss. However, ethical concerns, tumourigenicity (producing tumours), and the risk of graft rejection have limited the clinical application of hESCs.
Fetal neural progenitor cells
Fetal neural progenitor cells have also been explored as a potential therapeutic option for ALS. These NPCs possess self-renewal capabilities and the potential to differentiate into astrocytes, neurons and oligodendrocytes. Transplantation of fetal NPCs into ALS rodent models has demonstrated both safety and therapeutic potential, with evidence of survival, differentiation, and the release of neurotrophic factors that delay motor neuron death.
In 2009, the U.S Food and Drug Administration approved a phase 1 study to evaluate the safety and tolerability of surgical delivery of stem cells for the treatment of ALS. The trial involved 12 patients who received fetal-derived neural stem cells (NSI-566RSC) through intraspinal engraftment. The cells were injected into various sections of the spinal cord, 10 injections at a dose of 100,000 cells per injection. All patients underwent the procedure successfully, with no significant surgical or long-term complications. Following a monitoring period of six to 18 months, there were no indications that the stem cells caused toxicity or harm to the patients.
Induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) are derived from adult somatic cells through genetic reprogramming with special factors, and offer a solution to the ethical concerns and immune rejection issues associated with other stem cell types. iPSCs have been successfully differentiated into motor neurons both in vitro and in vivo. Transplantation of iPSC-derived neural progenitors engrafted in the adult spinal cord has demonstrated survival in high numbers and secretion of neurotrophic factors that modify the toxic environment.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs), derived from various adult sources such as bone marrow and adipose tissue, have also gained attention in ALS cell therapy. Notably, bone marrow-derived MSCs (BM-MSCs) have demonstrated the potential to differentiate into neuron-like cells, oligodendrocytes and astrocytes, both in vitro and in animal models of ALS, indicating their potential for neural repair. In addition to their regenerative properties, MSCs exhibit immunomodulatory and anti-apoptotic properties: these are properties that regulate immune response and prevent cell death by secreting various cytokines, growth factors, and extracellular matrix proteins that contribute to neuroprotection and tissue healing.
In 2012, human clinical trials involving MSC transplantation in ALS patients showed 90 per cent, one-year survival rate after transplantation and a mean long-term survival rate of 40.17 months from diagnosis. These characteristics, along with their ease of harvest and the potential for allogeneic (donor cells) and autologous (patient cells) transplantation, make MSCs an attractive option for ALS therapy.
Out of all the stem cells mentioned earlier, human iPSCs have unique advantages when compared to others. hiPSCs can be obtained from blood and urine, making them more accessible and easier to utilize in clinical treatment. Despite all these encouraging findings, further research is needed, and underway, to optimize stem cell-based therapies for ALS. The limited availability of stem cells for transplantation remains a challenge, particularly when considering the large-scale therapeutic requirements in human patients.
Promising news
Recently, Cedars Sinai developed a potential cure that passed the safety trial. The treatment involves combined stem cell and gene therapy for ALS using support cells and GNDF protein that can move past the blood-brain barrier to protect diseased motor neurons in the brain and spinal cord. “We were able to show that the engineered stem cell product can be safely transplanted in the human spinal cord. And after a one-time treatment, these cells can survive and produce an important protein for over three years that is known to protect motor neurons that die in ALS,” said Clive Svendsen, PhD, Professor Cedars Sinai.
Ongoing stem cell research efforts continue to unravel the mysteries surrounding ALS. These different approaches are working towards improved treatment strategies and, ultimately, a cure for this devastating neurological condition.
Peace Chukwu
Latest posts by Peace Chukwu (see all)
- New turn for stem cell therapy: How tiny messengers are about to change the game - June 18, 2025
- Menstrual stem cell implication in endometriosis - February 6, 2025
- Menstrual stem cells and their promise - January 10, 2025






Can stem cells used for treating ALS be extracted from a donor body?
Here is a good answer to your question. https://www.alscenter.cuimc.columbia.edu/research/als-stem-cell-core-program#:~:text=The%20ALS%20Center%2C%20in%20partnership,as%20cellular%20models%20of%20disease.