One of the major limitations of using stem cell donors is the challenge of finding an adequate match, which is why I always get excited about therapies that use a patient’s own tissue. But why is the issue of a “match” such a big deal? You may have come across terms like “graft versus host disease” and “life-long immunosuppressive therapy,” but what do these terms really mean? And why is it so difficult to find a “match” in the first place? I thought it was time for a deep dive into the biology of tissue and stem cell transplantation.
We know that the immune system helps us fight off infections and remove damaged or cancerous cells. And with the exception of autoimmune diseases, it does a pretty good job of leaving our healthy cells alone. But how does the immune system recognize “self” from “non-self”?
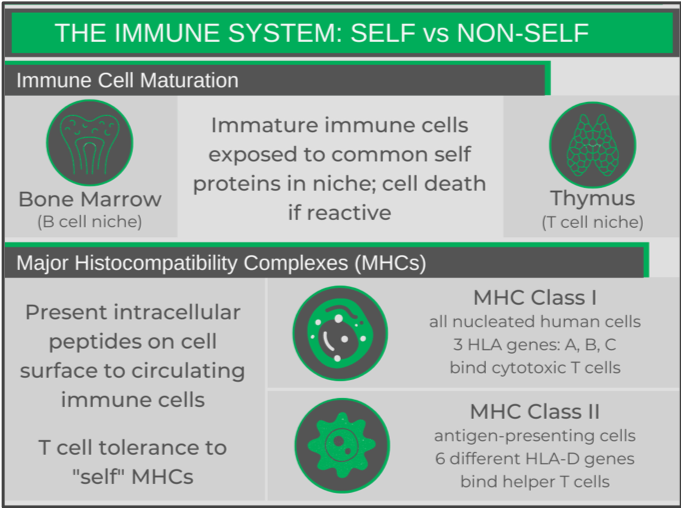
There are two main mechanisms that help the immune system distinguish self from non-self. One involves the way in which B and T cells develop (see my immune system primer post for more info on these cell types). While in their respective niches, immature B and T cells undergo V(D)J recombination, a process that allows for the random mutation of DNA during cell division in the sequences that encode their pathogen-recognizing receptors. This allows the immune cells to develop a diverse range of receptors towards pathogens (e.g. bacterial and viral proteins). By chance, some of these randomly generated receptors may recognize self-proteins. To ensure that these cells do not reach maturity and get released into circulation, while in their niches, the maturing B and T cells are exposed to various critical self-proteins. If the receptor recognizes any of these proteins, an auto-destruction pathway is triggered, destroying the cell.
Every cell in the human body (except red blood cells) expresses surface molecules called Major Histocompatibility Complexes (MHCs), that present small portions of intracellular proteins (peptides) on the surface of the cell to circulating T cells. The T cell receptors need to recognize both the MHC and peptide portions of the complex. T cells have a learned tolerance to an individual’s MHCs and normal peptides, so will not activate when they encounter a healthy cell. However, when a foreign peptide (e.g. from a viral protein) is presented, the T cell recognizing that specific MHC-peptide complex will activate, and kill the infected cell.
Each class of MHC has several “subtypes” (Human Leukocyte Antigens, HLAs), which are very specific to the individual. An individual will have two copies of each HLA gene (alleles), having inherited one from each of their parents. However, there is a ton of variability in each of these HLA genes – one has over 2,400 alleles – and an individual will only have one or two.
In fact, the diversity in the HLA genes is so large that no two individuals (with the exception of identical twins) will have the same set of MHC molecules.
This HLA variation is the issue when finding a transplant match. While the genetic variations may not change the function of the HLA protein, they are subtle enough for a T cell receptor to see a donor’s HLA protein as foreign. So how are we able to have any success in organ or stem cell donation?
Different organ transplants seem to have different requirements as to how stringent the matches are. For example, kidney and stem cell transplants seem to need the highest degree of matching; whereas, a corneal transplant doesn’t seem to be affected by HLA matching at all in some cases. Some HLA genes have less variability than others and are considered less important when trying to find a match, so the focus is really on making sure the most immunogenic HLA genes match. Furthermore, only certain parts of the gene play an important role in being immunogenic.
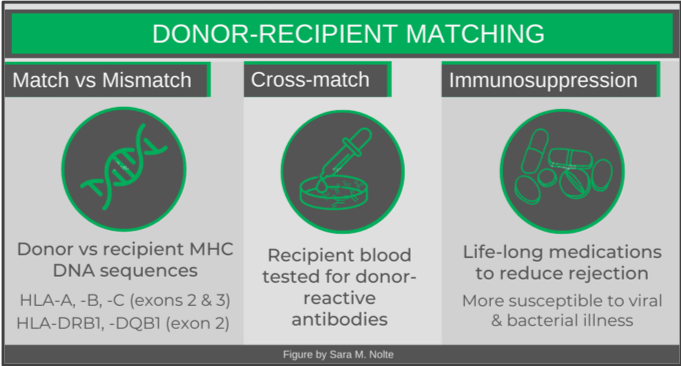
The first step in finding an appropriate match is identifying and comparing the most immunogenic HLA gene sequences of the donor and recipient (DNA is obtained most commonly via cheek swab). While there are a number of matching algorithms, most commonly 10 different HLA genes (five different genes, two copies each) are examined. Specific parts of each gene’s sequence are compared. A 10/10 match is ideal, as even a single mismatch (9/10) can be associated with graft versus host disease.
Once a donor-recipient match has been identified, cross-matching is then performed. This assesses whether the recipient has developed any circulating antibodies to a donor’s HLAs. Antibodies may have developed as a result of a previous transplant, blood transfusion, or even during pregnancy. If there is no recipient reactivity, the transplant may proceed.
The final step in ensuring a successful organ or stem cell transplant is for the recipient to be on life-long immunosuppressive therapy. While a 10/10 match does significantly reduce the chances of graft failure, there are still numerous donor HLAs that are not identical to the recipient and are recognized as foreign by the recipient’s T cells. There are various types of immunosuppressive medications that will prevent different actions of immune cells. Common mechanisms of these drugs include preventing the expression of cell-signalling molecules, inhibiting cell division, or reducing the mobility of immune cells. Patients are often on a combination of medications to improve the effectiveness of the immunosuppression.
The problem with these medications is that they are not specific to immune responses being generated against the donor tissue, but suppress the immune system as a whole. Patients will lack appropriate immune defences against bacterial and viral infections – meaning they can get very sick (or even die) from infections that usually just make you feel unwell for a week or two.
Clearly, we can see two main issues with the way things currently work: 1) the difficulty in finding appropriate matches; and 2) ongoing need for immunosuppressive therapy.
The best solution to these problems (since not everyone has an identical sibling) would be to find ways to use an individual’s own cells/tissues, ensuring identical HLAs. This is why we get so excited about new developments in stem cell technology and genetic engineering. For people undergoing stem cell transplants for genetic conditions, being able to replace the missing or mutated genes in their own stem cells (either from cord blood or bone marrow) via genetic engineering is clearly a game changer. Similarly, the ability to generate an organ or tissue graft from induced pluripotent stem cells (iPSCs) harvested from their healthy tissue are exciting opportunities for when a failing or damaged organ needs to be replaced (e.g. kidney, liver, cornea).
In the meantime, we’re still largely dependent on stem cell and organ donors. And given that April is organ donation awareness month, I would be remiss not to share how to register to be an organ donor in Canada (living or after death) based on your province of residence, or how to join Canada’s national stem cell registry. And here’s hoping that one day these registries will be obsolete!
Sara M. Nolte
Latest posts by Sara M. Nolte (see all)
- Ottawa researchers make tumour-marker negative cancers positive with oncolytic viruses - December 19, 2024
- Cell-based therapy approved as alternative to standard UCB transplant for hematological malignancies - June 21, 2023
- Finding the perfect match: breaking down the science of organ and stem cell transplant matching - April 18, 2023






Comments