 There are many ways the regenerative potential of cell transplantation therapy can be optimized to pave the route to the clinic. They include, but are not limited to, deciding which cells to transplant and looking at whether we should use fresh cells or cryopreserved cells.
There are many ways the regenerative potential of cell transplantation therapy can be optimized to pave the route to the clinic. They include, but are not limited to, deciding which cells to transplant and looking at whether we should use fresh cells or cryopreserved cells.
For example, there are different types of neural stem cells in the human brain. Very early in development (embryonic weeks 6-8), there are neuroepithelial stem (NES) cells, and they are responsible for building up the brain. Later, in the fetal brain (from embryonic weeks 10-12 and forward), the neural stem cells transform into a radial glial (RG) type of stem cell.
From her post-doc work, Dr. Anna Falk helped to distinguish these two human fetal-derived stem cells. She showed that they have different cell markers, that NES cells take a shorter time to proliferate compared to RGs, and that they have different differentiation profiles. (NES cells differentiate into 80-90 per cent neurons and 10-20 per cent glia cells, and RGs differentiate into 50 per cent neurons and 50 per cent glia.)
Dr. Anna Falk is now a Professor at Lund University and is affiliated with Karolinska Institutet in Sweden. She is also the Chief Scientific Officer of CCRM Nordic. In case you missed it, at last year’s Medicine by Design Symposium (December 9, 2024), she discussed one of her lab’s more current research endeavours: optimizing the storage, maintenance and manufacturing of transplantable induced pluripotent stem cell (iPSC)-derived NES cells.
In 2012, Dr. Falk and her collaborators showed that iPSC-derived NES cells do a better job in promoting recovery than fetal-derived RG cells after transplantation into mice with one type of spinal cord injury (SCI). In 2021, this experiment was repeated in rats with a different type of SCI in which there is cyst formation in the spinal cord. The research showed that NES cells shrink the cyst more than RG cells. For the rats that received no transplantation, the cyst only grew larger. Despite being stem cells, NES cells do not cause tumours once transplanted. For pre-clinical stroke models, it turns out that NES-derived cortical progenitors differentiate into mature cortical neurons post-transplantation and also do a good job in rescuing function.
In these studies, they transplanted fresh NES cells (cells were transplanted immediately after their production in the lab). But the results using fresh cells led to some transplants being really good and some that weren’t. A really good transplant is one where both the cyst length and volume shrank significantly, and not-so-good transplant outcomes were indicated by a cyst that typically remained around the same length and volume as it did pre-transplantation.
Variations in transplantation outcomes might be due to limitations in conducting different kinds of quality testing before transplantation when using fresh cells, as these tests may require lengthy procedures. A feasible quality test that can be done within a reasonable time on fresh cells immediately before transplantation is one that counts how many cells are alive in the sample. Cells pass quality control if there are more than 80 per cent live cells. There are other quality tests that can only be done when cells are no longer alive and take a certain amount of time that wouldn’t be feasible if transplanting fresh cells. One such test is immunostaining to test for differentiation potential of the cells. This procedure often takes a couple of days to complete.
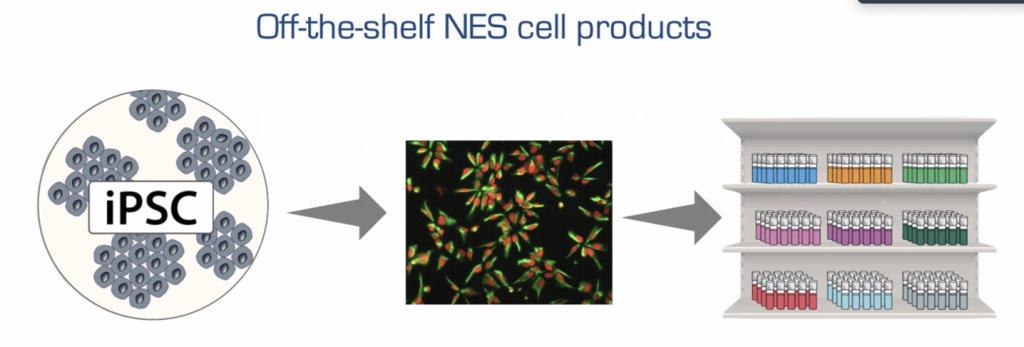
The researchers wanted to know how frozen-down, then thawed batches of NES cells would perform after transplantation. They knew that one way to create consistency between cell doses (and therefore outcomes) is to freeze down many ready-to-use batches of cells from the same starting source of fresh NES cells. Equal quantities of one NES cell source are distributed into hundreds of vials (doses) and then frozen. Therefore, each separate vial will have a known number of cells and cell quality will be similar between the vials. A subset of these vials can be easily set aside just before freezing to run more tests for identity, purity, genome integrity, quality and cell count, while the frozen-down cells sit in a cryopreserved state for later use. Quality control is improved with the cryopreservation process because from these additional quantification tests, you can decide if you have a good batch or not.
In terms of their effectiveness in transplantation outcomes, frozen and then thawed NES cells shrank the cyst by 84.4 per cent. Compare that to the previous transplantation study, in which the transplanted fresh NES cells shrank the cyst by 46.9 per cent. Not only that, but all transplants turned out well and were very similar. The identity, differentiation profile and viability between frozen and then thawed NES cells were the same as fresh NES cells. However, fresh NES cells could not be quality tested as much as frozen doses of cells. This result was also shown to be sustained for at least two years with minimal batch-to-batch variability.
The first part of these experiments was done using Good Manufacturing Practices (GMP), meaning there are strict procedures to follow: media and factors must be free of animal products and anything unknown; scientists wear sterile space-like suits in temperature-controlled clean rooms; and two operators are always present for every experiment – just to name a few. This is a significant feat toward being able to translate their pre-clinical results to the clinic, considering all in-human clinical trials must be GMP-compliant.
Although no loss of quality was seen between fresh and cryopreserved undifferentiated NES cells, the downside is that the more differentiated cells are, the more sensitive they are to cryopreservation. It will be more difficult to transplant cryopreserved mature neuronal cell types for cell therapy.
Nonetheless, Dr. Falk’s team demonstrated that if you are using undifferentiated NES cells for transplantation, stored, ready-to-go, precise cryopreserved NES cells are the way to the future. This type of batched storage ultimately has the potential to create ease-of-access when it comes to getting it to SCI patients in a timely manner.
Referring to the difference between RGs and NES cells, Dr. Falk said: “Neural stem cells come in different flavours.” In addition, there are so many different sources of stem cells that have been undergoing clinical trials for transplantation. For example, BlueRock Therapeutics uses embryonic stem cells to create dopamine neurons, and it’s these dopamine progenitors that get implanted into Parkinson’s patients as part of these clinical trials specifically.
Mesenchymal stem cells, which are not neural, have been used in clinical trials to treat neurodegenerative diseases like Alzheimer’s, ALS and Parkinson’s disease. Some pre-clinical trials are looking into creating spheres (clusters of neural stem cells) for implantation. The Okano lab uses NES cells, but instead of creating single cells, they create neurospheres. Each sphere contains 5,000-10,000 cells, which means that it might be more difficult to count the precise numbers of cells going into each transplanted dose, and they are implanted into pre-clinical SCI models. As it turns out, they are also doing a clinical trial using these neurospheres (registry # in Japan: jRCTa031190228).
Some of the reasons why different stem cell sources are being used by different labs can be attributed to how long the cells have been around. Embryonic stem cells have been around longer than iPSCs (1998 versus 2006). Also, people use whatever they have access to. For example, the cell lines BlueRock uses are well characterized, have been around a long time, and have undergone many successful tests already.
Having different companies use different cell types adds some competition and room for learning, which should foster the most optimal outcomes. Only time will tell. At ISSCR 2024 in Copenhagen, Melissa Carpenter revealed that by the end of 2024, more than 1,200 patients had undergone transplantation of human pluripotent stem cell-derived cells across a total of 116 approved clinical trials worldwide.
For more information on individual trials, different diseases that are being treated, and the hurdles we’ve overcome to get to this point, I recommend checking out this newly released review article published in January 2025. It is incredible to see the big picture. The Okano lab’s iPSC-derived NES cells have been approved for clinical trials in SCI (registry # in Japan: jRCTa031190228) – and these are with fresh cells. Perhaps one day we will also have off-the-shelf cryopreserved stem cells for consistent therapeutic transplantation outcomes.






Comments