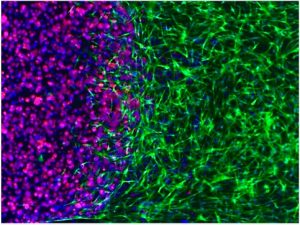
In vitro engineered complex tissue unit to study the interface between normal and tumour tissue, by Elisa D’Arcangelo.
It is the tricky task of tissue engineers to recreate tissues without instructions or authentic building blocks. Nevertheless, researchers have been imitating tissues in vitro from various cell types, according to their best judgment assembly protocols, through a lengthy process of iterative trial and error. The results have been impressive, though by and large quite far from the original.
It is from this inability to generate actual functioning organs from scratch that the idea of a “good enough” tissue has emerged.
While it may sound bizarre to think of lung, kidney or liver tissue as merely “good enough,” this concept is what allows tissue engineers to get (some) sleep at night: lab-grown tissues do not need to be “the real deal” – they just need to allow for certain biological processes to take place, and these processes can thus be studied outside of the body.
In fact, engineered tissues are being grown as functional units to replace human tissues, as models to understand how healthy and diseased tissues work and as testing platforms for drug screening. All of these applications rely on lab-grown tissues not to be perfect, but rather to simulate certain key biological processes observed in actual tissues.
If this all sounds a little complicated, that’s because it is: organ development is mind-bogglingly complex and, thus, it is not surprising that researchers are aiming to achieve more and more complexity in their engineered tissues. This comes in the form of a myriad of additional cell types to be incorporated, various three-dimensional protein meshes to fool cells into creating spatial complexity, and even artificial fluid flow and tissue stretching forces to shape cells into their correct forms.
Such approaches, however, stand in direct opposition with a fundamental scientific best practice: namely, the pursuit of simplicity. Why make a really complex tissue if the specific biological process you want to study can occur in a much simpler one? For example, if the effect of one cell type’s secretions on another can simply be mimicked by allowing the cells to share their liquid surroundings, there is no need to devise a system where both cell types could physically interact with each other.
The tightrope walk between simplicity and complexity has, in the past few years, become a major point of discussion, not only among tissue engineers, but the wider biology community.
On April 26, 2021, Cell Press held an online symposium session precisely on this topic, asking “How simple is complex enough?” The panel of experts mostly agreed that the complexity of an engineered model tissue directly depends on what information one wants to obtain from it. For example, assessing the effect of a drug library on cell proliferation probably requires far less complexity than modeling a process such as organ development.
There is, however, the elephant in the room, which seldom gets acknowledged: researchers can only guess a priori, based on prior knowledge, as to what level of complexity is required to achieve the manifestation of the desired biological processes in their models. Thus, the lines between what is a minimum requirement, and what is an unnecessary and even confounding surplus become blurred, leading to large numbers of engineered tissues that are hardly useful in the long-term. But, thankfully, “good enough” tissues are being made (think for example of experimental procedures that have made headlines, such as skin grafts, replacement tracheas and replacement bladders) and the definition of what good enough means is becoming ever more precise.
This is happening especially thanks to novel technologies, including single cell RNA sequencing, with which we can explore the variety of cellular phenotypes present within a tissue with an unprecedented level of detail or imaging mass cytometry, which allows a spatially resolved view of proteins expressed in a tissue. Having such benchmarks to aim for will undoubtedly steer the search for complexity in the right direction.
In late 2020, “Is it time for Reviewer 3 to Request Human Organ Chip Experiments Instead of Animal Validation Studies?” was published. In it, Prof. Don Ingber, a chief contributor to the scientific field of tissue engineering and organ-on-a-chip engineering, asked what many scientists are wondering: If engineered tissues are beginning to be good enough for certain questions in biology, do they have the potential to be better than animal models currently being used?
Traditionally, and precisely because biologists could not create complexity successfully in vitro, the gold standard for observing “the real deal” in terms of biological processes has been animal studies. Do you have a scientific theory that showed promise in vitro? You will have to prove your theory holds up in a living organism; if it does not, your initial results are simply due to the fact that you were working with an overly simplistic in vitro model.
Sadly, looking back on how poorly these animal models have performed in terms of successfully identifying treatments that also work on people, the consensus has emerged that they are, on aggregate, not particularly good models of human complexity.
So, does the advent of complex engineered tissues imply that animal models of disease are obsolete? The conclusion reached here, which echoes that of most scientists, is that there will still be many years of animal testing ahead of us, precisely because in vitro-generated complexity still needs to be bench-marked.
Truthfully, we are far away from growing ‘true’ organs in the lab. What researchers are striving to understand with increasing precision, is how to set the bar for good enough tissues – this will be the true game-changer for disease modeling going froward.
Elisa D'Arcangelo
Latest posts by Elisa D'Arcangelo (see all)
- About tissue regeneration, fibroblasts and reindeer - December 27, 2021
- It’s all about the Niche! Take-aways and highlights from TMM 2021 - November 25, 2021
- “Good Enough” tissue engineering - May 11, 2021






Comments