Almost two years ago, I wrote a post about how scientists were looking for new immunotherapies to improve immunologically “cold” tumours’ responses to existing therapies. And while the findings of that article were very promising, tumour evasion of the immune system continues to vex cancer researchers.
A recent study, led by Dr. Michele Ardolino from the University of Ottawa, has uncovered yet another way that cancer cells slip past immune surveillance. Their work examines the mechanism behind puzzling observations that the natural killer (NK) cells of mice with tumours possess and are supressed by the protein PD-1, despite not making it themselves.
Before we get too far into the study details, here are some relevant immunology tidbits worth knowing:
- Natural Killer (NK) cells: are cytotoxic white blood cells; they cause apoptosis (cell death) in their target cells. As part of the innate immune system, they have the advantage of not requiring antibody-mediated activation like other immune cell types (so they can attack infected/damaged cells missed by other immune cells).
- PD-1 (programmed cell death protein 1): an immune system checkpoint protein, usually found on T and B cells. When it binds its ligand (PD-L1), a signal cascade to turn “off” is initiated, and the immune cell stops attacking/killing other cells. It is well established that cancer cells take advantage of this signalling pathway, by expressing PD-L1 to suppress host T cells. Anti-PD-1 antibodies are an immunotherapy used in these cancers to block PD-1 from interacting with PD-L1.
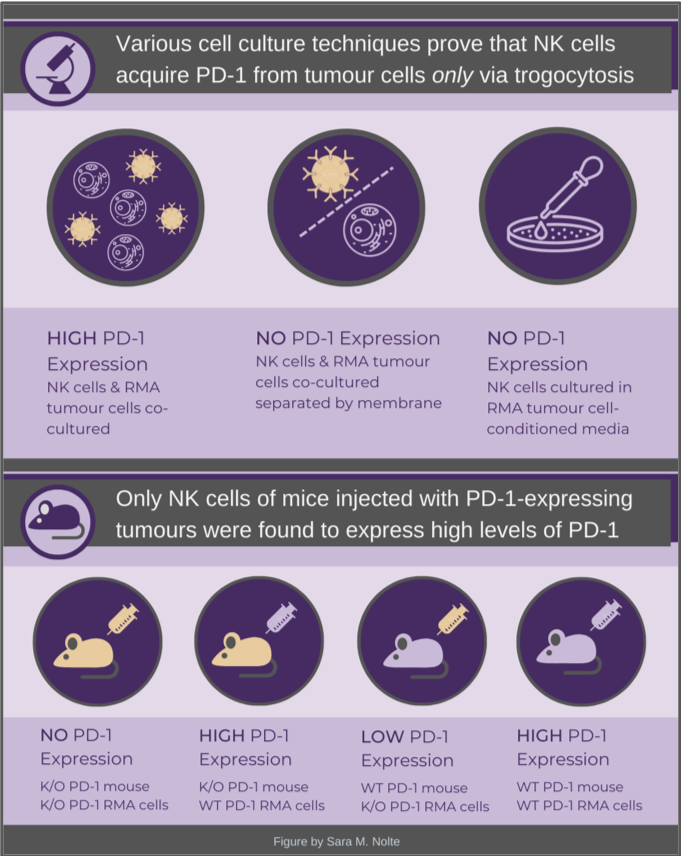
- Trogocytosis: the process by which a cell “nibbles” off a piece of another cell’s membrane, acquiring parts of the membrane and associated membrane-bound proteins (which remain intact and functional). Immune cells frequently engage in trogocytosis, acquiring proteins that mediate their response. (See also this in-depth review paper).
The research team had previously identified that NK cells were suppressed by the PD-1 pathway. While other researchers had made similar observations, these findings remained at odds with the fact that no one had been able to identify endogenously expressed PD-1 mRNA or proteins. It was clear to Dr. Ardolino that the NK cells must be getting PD-1 from the tumour cells – the question was how?
Knowing that NK cells sometimes engage in trogocytosis, after which their activity is frequently suppressed, and seeing recent reports on NK cells obtaining other proteins from cancer cells this way, Dr. Ardolino wondered if maybe trogocytosis could be the mechanism by which the NK cells acquired PD-1.
Using various in vitro models, the group demonstrated that cell-to-cell contact between NK cells and tumour cells (RMA and leukemia cell lines) was required for NK cells to express PD-1. By tagging a fluorescent protein to tumour cell PD-1, they were actually able to observe NK cells engulfing chunks of tumour cell membrane (which included PD-1) via fluorescent live-imaging, within minutes of being introduced to the culture. When NK cells, pre-treated with inhibitors of trogocytosis, were subsequently co-cultured with tumour cells, they had a strong reduction in their ability to acquire PD-1. Together, these results confirmed that NK cells acquired PD-1 from tumour cells via trogocytosis.
By injecting PD-1 wildtype or knockout mice with either PD-1 wildtype or knockout tumour cells, the researchers found that NK cell PD-1 protein expression occurred only when the tumour cells expressed PD-1. The tumours were able to grow much larger when they expressed high levels of PD-1, compared to the knockout tumours. When the mice were also treated with a PD-1 inhibitor, the tumour growth rates were similarly low. These findings confirm that PD-1 is acquired by NK cells, is inhibiting their cytotoxic effects, and this suppression could be reversed by treatment with commonly used PD-1 inhibitors.
The authors also assessed the bone marrow of patients with multiple myeloma for evidence of NK cell trogocytosis of PD-1. Using flow cytometry, they were able to identify a consistent population of NK cells that expressed a surrogate marker of trogocytosis (CD138) and PD-1, confirming their murine model findings. However, this final part of the study does not address the functionality or clinical relevance in cancer patients.
I think it would be very interesting to know if the expression of PD-1 on a patient’s NK cells correlates with their clinical response to treatments – do those with higher levels of PD-1 do more poorly? If so, does this suggest that there is a new subset of patients that would benefit from treatment that includes a PD-1 inhibitor? Are these patients already receiving a PD-1 inhibitor, and are they now considered refractory treatment? Do they require an additional component to their therapy, perhaps an inhibitor of trogocytosis? These are questions that the authors are also keen to address in future translational studies. For now, our cancer immunotherapy appetites will just have to settle for this new morsel of research!
Sara M. Nolte
Latest posts by Sara M. Nolte (see all)
- Ottawa researchers make tumour-marker negative cancers positive with oncolytic viruses - December 19, 2024
- Cell-based therapy approved as alternative to standard UCB transplant for hematological malignancies - June 21, 2023
- Finding the perfect match: breaking down the science of organ and stem cell transplant matching - April 18, 2023






Have you studied Lineage Cell Therapeutics’ VAC platform?
I have not, but your comment is super relevant, as I’ll be covering various types of cancer immunotherapies in my next post (hopefully coming September) – I’ll be sure to include vaccines! And if you think this platform deserves a post of its own, let us know!