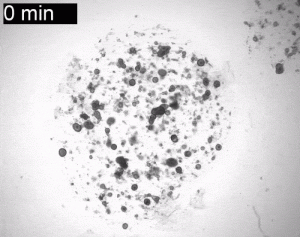
Crying organoids, just like real tear glands. From the lab of Dr. Hans Clevers at the Hubrecht Institute. Shared with permission.
Regenerative medicine news under the microscope is a new monthly feature highlighting big stories in stem cell research. I will sample the latest and greatest findings in recent press and package them into a single post.
You can expect these blogs to be published at the end of each month under regular circumstances; however, the past few months have yielded some remarkable research findings, so this first post will bring us up-to-date. The next post, which is slated for the end of this month, will be your April highlight.
I’ve come across important work on everything from COVID-19, tear glands, aging and much more! Let’s start with the topic on everyone’s mind: the virus.
COVID-19: The latest developments in research on heart damage and human amniotic cell-based treatments.
There’s been some controversy as to whether the effects of COVID-19 on heart tissue, including myocarditis (inflammation of the heart muscle) are direct or not. A direct effect would mean that the virus is infecting the muscle tissue itself, while an indirect effect could potentially be due to inflammation occurring systemically. Thrombosis was also suggested as an indirect contributor.
A recent U.S. collaboration has revealed that the direct infection of cardiomyocytes (heart muscle cells) is indeed possible and leads to myocardial tissue damage. The researchers established a system to model myocardial pathologies caused by SARS-CoV-2 using engineered heart tissue, demonstrating virus-induced deficits in contractility, sarcomere assembly, cytokine production, and even cell death.
An interesting point to note here is that SARS-CoV-2 was not found to directly infect cardiac endothelial cells, fibroblasts, or macrophages; cardiomyocytes are the primary target identified by this paper.
This study might help to settle the controversy and, perhaps just as importantly, offers up a model system for further study of SARS-CoV-2 in myocardial tissue.
The next COVID-19 highlight is a recent review article on what’s known about human amniotic therapies for SARS-CoV-2 infection, as of March. If you’ve been keeping up with the latest on the subject, feel free to skip this read. However, if you’re looking to catch up, Riedel et al. have you covered.
Vision science: Making organoids cry.
This next story is nothing to blink at.
While many of us don’t think much about our tear glands on any given day, they are critical organs that keep our eyes well-lubricated. Why is this important? Well, when tear glands malfunction, dry eye syndrome might result, leaving patients with irritated eyes, blurred vision and, in severe cases, corneal damage.
At the moment, mild cases of dry eye syndrome can be treated with eye drops, but serious cases may require surgery. Surprisingly, the limited nature of these options is due to the fact that we don’t know much about lacrimal glands, or tear glands. My next research highlight tackles this issue.
Bannier-Hélaouët et al. developed both human and mouse lacrimal gland organoids; showed that the gene Pax6 is a must for differentiation of this cell type; offered up a single cell atlas of both human lacrimal gland organoids and dissected tissue (scRNAseq buffs: they also conducted an RNA velocity analysis); and, proved that their organoids can cry, just like the real organs. To see what this looks like, look no further than the gif at the top of this blog post! The organoids are the spheroid structures shown. Our tear glands respond to a mix of neurotransmitters released by our bodies when we’re about to cry. The researchers replicated this effect by simply applying the neurotransmitters to their organoids, causing the structures to fill up with tear fluid, thus expanding! Why do they expand? The organoids themselves don’t have structurally sound ducts to outlet the fluid so, instead, the tears are trapped inside.
To top it all off, the researchers transplanted their dish-grown human tear glands into mice, and showed that they seem to self-organize into the host and engraft successfully. They even see the formation of a structure resembling a duct, and the accumulation of tear proteins therein. Though the transplant evidence was preliminary, more studies will reveal how safe and how truly functional such an approach might be. For further reading, check out this article by Nature’s Heidi Ledford, which features additional details concerning how salivary gland transplants may set the stage for tear glands in the future, and just what exactly crocodile tears have to do with this story!
Advanced gene editing in the fight against rapid aging.
Aging is always a hot topic in stem cell research and science fiction alike. This next highlight covers significant findings in this field published by Nature this past January.
You may have heard of Progeria, a disease which speeds up the aging process so dramatically that patient lifespans average out to a tragic 14 years. This devastation is the result of a genetic mutation that codes for a toxic protein called progerin. Though some treatment options do exist, a true fix involving reversal of the damaging mutation itself has not yet hit the clinic. Enter Koblan et al., who aim to change this.
Their approach involves a technique called base editing, which allows for the targeted conversion of a DNA base (for instance, a mutated site) without necessitating a double-stranded break in the DNA molecule. Why is this interesting to us? It’s both more efficient (almost ten times more, in fact) than the usual gene editing tool CRISPR-Cas9, and it also produces fewer genetic errors. CRISPR-Cas9 editing does involve a double-stranded DNA break, and when this break is repaired by the cell, random DNA bases are sometimes incorporated or removed. This fundamentally alters the genetic sequence in ways not necessarily intended, and which can become problematic. That’s why base editing is so strong – this happens far less often. For further reading on this topic, click here.
Using base editing, the researchers were able to significantly improve outcomes in mouse models. The result? Dramatically reduced progerin levels, a rescue of the normal gene product (lamin A, a critical scaffolding protein found in the nuclear envelope), normal vascular profiles that contrast the arterial pathologies usually observed in progeria patients, and a doubled mouse lifespan – all accomplished by a single round of gene editing, or one injection. The editing was also successful and highly efficient in patient fibroblast cells (in vitro). These editing strategies were undertaken in fairly young mice, however, at three and 14 days old. Luckily for us, a 14-day old mouse is technically equivalent to a 5-6 year-old human being, in terms of physiological maturity! That lays promising groundwork for treatments that might be effective not only when administered at very early ages (while still a baby, for instance) but also for older patients, as well.
Though this treatment isn’t quite ready to hit clinics just yet, and will require further optimization in terms of delivery strategy, treatment timing, dosing and overall safety, tools like these may have the potential to serve as strong weapons in the fight against rapid aging.
Experimental repair of non-penetrating human spinal cord injuries using patient stem cells
In a joint, 13-case effort between Sapporo Medical University in Japan and Yale University in the U.S. published this February, researchers brought the world one step closer to the prospect of central nervous system injury repair using patient-derived stem cells.
Bone marrow derived stem cells were injected intravenously into patients with non-penetrating spinal cord injuries in these trials. Penetrating injuries would include those caused by stabbings, gun shots, or missile attacks, and are not included in the study.
The patients in these cases sustained their injuries from events such as a fall or exposure to minor trauma, and suffered from symptoms including a reduction or loss of motor function and coordination, sensory capabilities, and bladder/bowel function.
More than half of those patients showed significant improvements within weeks of the stem cell injection and, remarkably, no serious side effects were reported.
It is important to note that the study was not blinded and that there were not any placebo controls. Thus, senior authors on the paper emphasize the preliminary nature of this work, that additional studies will be required, and that those additional studies may take years. Still, this research is reason for optimism in the field. Similar techniques are usually applied successfully in animal models, and Honmou et al.’s trial marks the potential transition of this concept from the research lab to the clinic.
Slaughter-free meat enjoyed by club patrons in Singapore
Though this isn’t quite a regenerative medicine story, it’s certainly an eye-catching headline worth mentioning here! In a world first, Singapore’s food agency has approved lab-grown, in vitro-cultured chicken for consumption by the public. According to Nature, the meat hit a club called 1880, with more than 200 servings already ordered.
Grown in bioreactors, these meat products begin as small cell populations obtained from donor animals and cell banks. They then proliferate in specialized media to form a minced meat product, priced similarly to organic chicken. The minced meat is then made into nuggets and marketed as chicken bites.
Eat Just, the group behind the cell-based meat in this story, is currently developing a scaffold upon which they aim to grow products more similar in texture to solid chicken breast, or perhaps even a marbled steak.
The most impressive aspect of the lab-grown meat endeavour is that the majority of these companies (and there are quite a few: at least 80 worldwide!) face technical challenges when attempting to scale-up their meat production in a way that is financially feasible. Eat Just, for instance, may not turn a profit for another 3-6 years, depending on how soon they can scale their facility from its current 1,000-litre capacity to upwards of 50,000 litres.
For more on lab-grown meats, check out a previous Signals blog post from November 2020: The secret sauce of lab-grown meats.
Additional recommendations
I wish I could have fit them all into the main body of this post, but there were just so many great stories in the first quarter of 2021 that it was impossible. That being said, here are some additional papers I’d highly recommend. They’re a mix of both clinical, regenerative medicine-themed studies, plus some general stem cell work that will no doubt lay the groundwork for important clinical research in the future. They received a ton of buzz from the community, so without further ado, here they are:
Cortical organoids: Mapping in vitro developmental time points to the real thing. Gordon et al. in Nature Neuroscience.
Modelling early development in a dish. Liu et al. in Nature and Yu et al. in Nature.
Naïveté lost, but not quite primed: An intermediate stem cell state. Hoogland and Marks in Cell Research.
Linking exercise to the immune system. Shen et al. in Nature.
Managing the mitochondria may be critical for neuronal replacement therapy. Russo et al. in Cell Stem Cell.
Boosting the aging immune system: Are drug therapies on the horizon? Dong et al. in Nature.
Stay tuned for my next post at the end of this month! I’ll round up some of April’s biggest stories and summarize them right here.






Comments