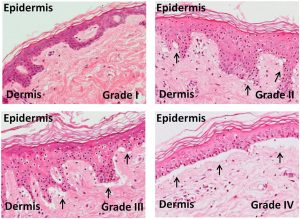
Micrographs show grading of skin in graft-versus-host disease. An increase in vacuole formation (arrows) between the dermis and epidermis is observed in higher grades, with a separation of the dermis from the epidermis in Grade IV. CC-BY 4.0.
For the first time, mesenchymal stromal cells (MSCs) can be used in routine clinical practice under the nod of regulatory approval in the United States. The MSC product known as Ryoncil was made available to patients in March 2025 following a historic decision from the U.S. Food & Drug Administration (FDA) to approve its first MSC therapy.
Ryoncil is the brand name for the MSC product remestemcel-L, manufactured by the Australian company, Mesoblast Ltd. It will be used as a second-line treatment for pediatric patients over 2 months of age who suffer from acute graft-versus-host disease (GVHD), a devastating condition that arises as a common complication of bone marrow transplants.
People with blood cancers, such as leukemia, are typically treated with a bone marrow transplant. The transplant procedure is intended to replace the recipient’s bone marrow tissue with healthy donor cells. However, in some cases, the donor cells recognize and attack the recipient’s tissues, causing the development of acute GVHD. The condition is characterized by severe inflammation and tissue damage impacting the skin, liver, gut, and other organs.
Individuals with GVHD are treated with anti-inflammatory steroids, but approximately half of these patients do not respond to the treatment, leading to severe disease and high mortality rates. Prior to the FDA’s approval of Ryoncil, there were no additional treatment options for children with GVHD after steroid treatment regimens failed. Ryoncil, an allogeneic cell product (i.e. sourced from genetically unmatched donors), will now be used to treat these steroid-refractory pediatric patients via intravenous infusion.
While the FDA approval is a major landmark for the MSC field, the path to this approval was long and tumultuous. The journey was ignited by a seminal case report published in 2004 in which a pediatric GVHD patient was treated with MSCs, leading to a full recovery. Following this initial report, decades of clinical research produced a series of notable failures in large Phase III clinical trials, drawing skepticism from researchers and investors alike.
It turns out that MSCs are a somewhat mercurial cell type. Once thought of simply as “stem cells,” MSCs are now known to serve diverse roles in regulating tissues, altering their cell behaviour on cue to help maintain homeostasis. In particular, MSCs can respond robustly to inflammation, and their strong anti-inflammatory properties have been exploited in clinical investigations for a wide variety of diseases, including GVHD. However, the complexity of MSCs as living drugs has led to a host of challenges, including in understanding more precisely the mechanisms through which these cells work, as well as in the development of manufacturing and quality control measures to ensure that their therapeutic power is harnessed effectively.
Pivotal data shared at International Society for Cell & Gene Therapy North America Town Hall
For Mesoblast, it took three application attempts to get approval for Ryoncil. In the first two attempts, the FDA cited concerns over the company’s supporting data, namely on efficacy and on the in vitro potency assay used to demonstrate consistent anti-inflammatory functions across different batches of Ryoncil. But it was third time lucky for the company, with the FDA approving Mesoblast’s application in December 2024.
In March 2025, Mesoblast’s CEO, Dr. Silviu Itescu, spoke at the International Society for Cell & Gene Therapy (ISCT) North America Town Hall and shed light on the data that paved the way for the pioneering FDA approval.
For historical context, Mesoblast had acquired an MSC product called Prochymal from Osiris Therapeutics back in 2013. Osiris completed a Phase III clinical trial in 2009 using Prochymal to treat adult and pediatric patients with acute steroid-refractory GVHD, but the trial failed to achieve its predetermined endpoint for efficacy.
A subgroup analysis showed that the MSC treatment was more effective in children compared to adults, and these data even served as the basis for a conditional approval from Health Canada, in 2012, for Prochymal to be used in pediatric GVHD patients. However, the product was never used in Canada, and it was subsequently bought by Mesoblast the following year.
Researchers at Mesoblast and in the MSC community postulated that the failed Phase III trial was due to issues with the clinical trial design, lot-to-lot inconsistency of the MSC batches, and overall low potency of the cell product.
To establish a higher-quality MSC product, Mesoblast needed to make improvements to the cell manufacturing process. The company changed their contract development and manufacturing organization (CDMO) and spent over a decade tailoring the manufacturing process to produce Ryoncil.
Dr. Itescu presented data showing that Ryoncil displayed superior potency compared to Prochymal, as demonstrated through inhibition of T cell activation in vitro. Furthermore, this corresponded to substantial improvements in the survival of children treated with Ryoncil versus Prochymal through the U.S. Expanded Access Program.
Bolstered by the cell manufacturing improvements, Mesoblast completed a pivotal Phase III clinical trial in pediatric patients with acute steroid-refractory GVHD, the results of which were published in the Biology of Blood and Marrow Transplantation journal.
The trial demonstrated an overall response rate of 70 per cent at day 28 follow-up, a value that was considerably higher than the 45 per cent response rate expected for other treatments. In his talk at the ISCT North America Town Hall, Dr. Itescu reported long-term follow-up data showing a 51 per cent survival rate at year two and a 49 per cent survival rate at year four.
Importantly, the causes of death in most children were not due to GVHD itself, but rather due to other bone marrow transplant complications or due to a return of the cancer for which they were initially treated. Furthermore, the trial demonstrated that Ryoncil was safe – an important finding given that children with GVHD are at high risk of adverse events.
To receive FDA approval, Mesoblast also needed to demonstrate that the in vitro potency assay used for quality control of Ryoncil batches was relevant to the proposed anti-inflammatory mechanism of action for the cell therapy. Dr. Itescu reported data demonstrating that the ability of Ryoncil to suppress T cell activation in vitro was strongly correlated to a reduction in circulating activated T cells in patients treated with the same MSCs. These data helped to confirm that Ryoncil is fit for targeting important cellular mediators of GVHD and that the potency assay measured a clinically relevant function of the MSCs.
Based on Mesoblast’s body of data, the FDA ultimately approved Ryoncil. The recommended dosing for the MSC product is two million cells/kg body weight, administered twice weekly over the course of four weeks. If a complete response has not been achieved after four weeks, Mesoblast recommends an additional month of treatment.
The cost per infusion of Ryoncil is US$194,000, totalling $1.55 million for a four-week treatment regimen, or $3.1 million for eight weeks of treatment. Dr. Itescu stated that pricing is in line with other approved cell and gene therapy products that offer similar survival outcomes. He further emphasized that, using conventional treatments, the economic cost of treating a child who ultimately dies of acute GVHD is approximately $2.5 million.
Mesoblast recently announced that nine commercial insurers have published positive insurance coverage decisions for the drug. The planned roll-out for Ryoncil will prioritize 45 high-volume treatment centres with previous experience in administering Ryoncil. The first three children were scheduled to receive the treatment at the end of March 2025.
Kevin Robb
Latest posts by Kevin Robb (see all)
- Mesenchymal stromal cell product joins FDA’s list of approved cell and gene therapies - May 22, 2025
- Lessons learned from market-approved mesenchymal stromal cell products - August 22, 2024
- A bench-to-bedside story of BlueRock’s investigational stem cell therapy for Parkinson’s disease - November 9, 2023






Comments