For decades, exosomes were seen as the cell’s garbage collection system. The understanding of their function was limited to cellular waste disposal, removing unwanted substances from the cell. Now, researchers are making the shift from using whole stem cells to exosomes, and they’re quickly becoming a new regenerative medicine favourite.
We know what makes stem cells special: their regenerative potential. However, we’ve begun to understand that their therapeutic benefits can be captured by their secretion. Among the many therapeutic molecules that they secrete, none of them in isolation or combination have been able to replicate the same effects as stem cell transplantation, except exosomes. They are tiny vesicles with a lipid membrane that contains chemical signaling molecules.
These molecules could be proteins, lipids, regulatory RNAs and other metabolites depending on their cellular origin. By delivering their content to other cells, they can influence how those cells behave, even at a distance. This mechanism has been refined to a targeted drug delivery system, sometimes aided by engineering.
About exosomes
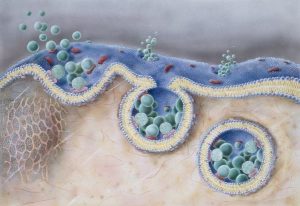
Exosomes are nano-sized particles measuring from 30-120nm, usually excreted as microvesicles into the extracellular space. They are secreted by all cells and are found in particularly large numbers in body fluids including saliva, urine, blood, bile, lymph and breast milk. Their specific composition depends on their cell of origin.
Due to their many benefits, scientists have figured out better large-scale extraction methods from therapeutic cells, such as stem cells. This, followed by repacking with desired regenerative products, holds the key to their application for diseases being studied, such as cancers, osteoarthritis, chronic conditions and autoimmune diseases.
Therapeutic benefits and how they work
Exosomes primarily function as signaling agents in cell-to-cell communication. They also mediate differentiation and deep tissue repair, carrying proteins, nucleic acid and lipids from their cell of origin to the target cells, which can be used in various cellular customizations. By acting as biocompatible cargo vehicles, they can influence cellular response and control organ function.
Their therapeutic potential is currently being explored in a lot of research and applications, including chemotherapy, cardiovascular, neurology and even dermatology. Studies analyzing their composition found a variety of miRNA strands like the miR-223, miR210 and miR-21 that can inhibit cellular death and make resistant cancer cells more sensitive to chemotherapy.
Exosomes reduce inflammation in various disease conditions by increasing anti-inflammatory cytokines and chemokines, and reducing the expression of pro-inflammatory cytokines.
Exosomes are also capable of recognizing self tissues and homotypic targeting (the preferential interaction with cells of similar or identical types), placing them at the forefront of precision medicine. Seeing how awesome they are, it didn’t take long for researchers to start engaging them as a drug delivery system.
How do stem cell exosomes specifically target cells?
Stem cell exosomes find and target specific cells in the body using a mix of natural signals, surface proteins, and special interactions with cell receptors. They have a built-in ability to act like delivery vehicles. Once inside the body, exosomes travel through the bloodstream and are guided by chemical signals released by cells in need. For example, injured or inflamed cells emit molecules such as CXCR4 and SDF-1a, which act as beacons to attract the exosomes.
Their outer layer also plays a big role. Special proteins and markers on their membrane help them recognize and latch onto the right type of cells. Scientists have taken this a step further by engineering the surface of exosomes with added ligands or receptors that can lock onto target cells even more precisely. In cancer treatment, for instance, exosomes can be loaded with chemotherapy drugs and sent directly to tumour sites. This focused delivery method is much safer than traditional chemotherapy, which affects the whole body and damages healthy cells too. Because of this precision, exosome-based drug delivery is becoming a more popular approach in medicine.
Adaptations/clinical translations
How scientists modify exosomes to make them work even better
Exosomes, on their own, already do a lot of impressive work in cell communication and therapy, but researchers have found ways to adapt them for better results. Since exosomes are delivery vehicles, think of it like upgrading a delivery truck. You can either load it with better cargo (what’s inside) or install a GPS module so it knows exactly where to go (what it targets). That’s essentially what exosome modification is about, and scientists usually divide this into two broad strategies: internal modification and surface modification.
- Internal modification – packing the right cargo
These tiny vesicles can be loaded with drugs, RNA, or even vaccines, making them miniature delivery systems that are naturally good at avoiding the immune system and reaching the right cells. Pretty handy, right?
Loading can happen in two ways:
Before the exosomes are even made, by modifying the parent cells (through things like transfection or co-incubation), so the resulting exosomes come pre-packed with your desired content.
After the exosomes are collected, using techniques like electroporation, freeze-thaw cycles, sonication (using sound waves), or even gentle mixing to insert the cargo.
For example, one team used electroporation to load doxorubicin (a chemo drug) into exosomes derived from dendritic cells to treat breast cancer. Another study used incubation and sonication to pack paclitaxel into immune-cell-derived exosomes to fight drug-resistant cancer cells. And in a different case, researchers loaded therapeutic let-7a miRNA into exosomes to target breast tumours. These methods are varied, but the goal is the same: deliver the right payload to the right place as efficiently as possible.
- Surface modification – getting exosomes to the right place
Now let’s talk about the outside. This is where researchers get creative with the exosome surface to improve targeting – essentially programming the exosome to knock on the right door.
How do they do it? A few different ways:
Genetic engineering: Scientists can tweak the parent cells so the exosomes they release have specific proteins or peptides on their surface.
Chemical linking: Using reactions to attach targeting ligands or peptides to the exosome membrane.
Electrostatic interaction: Playing with charge-based binding to modify the surface without permanent changes.
Magnetic nanoparticles: Yep, even magnets get involved to guide exosomes to certain tissues using external fields.
One striking example: a team engineered dendritic cells to express specific peptides (Lamp2b fused with RVG) on their exosomes, enabling them to cross the blood-brain barrier and deliver cargo directly to the central nervous system. In another study, researchers used chemical reactions to add tumour-targeting peptides to exosomes aimed at glioblastoma cells. And in yet another approach, exosomes were bound to a pH-sensitive fusion peptide-lipid complex using electrostatic interaction, enhancing how effectively they delivered their cargo inside target cells.
Over 150 clinical trials have been registered on ClinicalTrials.gov exploring how exosome therapy might help treat different illnesses, including lung conditions, infections and cancer. Out of these, 31 trials focus specifically on exosomes taken from stem cells, attesting to their ability to replicate the therapeutic benefits of stem cells.
Neurodegenerative diseases
The most common neurodegenerative diseases, Alzheimer and Parkinson’s, have been studied worldwide and the irreversible damage that occurs in these diseases is the prime reason therapeutic solutions seem elusive.
However, exosomes from oligodendroglial cells protect neurons from oxidative stress and have also been found to have significant healing effects on neurons. In an in vitro model of ischaemia, oligodendroglial exosomes transfer protective proteins such as catalase and superoxide dismutase (SOD) that exert beneficial effects on neurons. Analyses after exposure of neurons to these exosomes showed differential gene expressions, an increased action potential, increased firing rate and altered cellular signal transduction pathways.
Exosomes also play a role in repairing bone and cartilage (Osteochondral regeneration), which may support healthier aging by improving joint function and slowing tissue deterioration in conditions like osteoarthritis.
Advantages
One of the greatest challenges in regenerative medicine and stem cell therapy is procuring sufficient quantities for therapeutic applications in tissue engineering and stem cell transplants. Stem cell sources are limited, so to amass the desired quantities, stem cells need to be cultured for many generations, over an extended period.
This opens up a new set of issues. Stem cells can develop genetic abnormalities such as chromosomal alterations and loss of heterozygosity. Since they are pluripotent, defects could affect their self-replicating function, triggering uncontrolled growth and resulting in tumours that could be cancerous. This is especially concerning in highly pluripotent cells like embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), both of which have been shown to form tumours.
Exosomes, in contrast, are cell-free and non-pluripotent, therefore they are not capable of causing tumour formation by replicating. In addition, there is a higher probability of developing a neurodegenerative disease with stem cells than with exosomes.
There is also a reduced risk of organ damage compared to stem cell therapy from immune rejection. Since the exosomes originated from the recipient’s own body, they do not require long-term integration into the body like transplanted stem cells. They minimize risks related to immune rejection by promoting immune modulation, reducing inflammation and influencing tissue repair, basically encapsulating a greater part of the benefits of stem cells without the pitfalls. This is particularly helpful in immunocompromised individuals, such as those receiving chemotherapy.
Storage of stem cells is more complex. They are very sensitive to environmental conditions and are unstable. Therefore, storage happens through cryopreservation – constant freezing in liquid nitrogen at -196 °C. Exosomes, in comparison, are stable enough to be stored long-term at room temperature after lyophilization (freeze drying).
These advantages make exosomes an attractive, safer alternative for regenerative medicine applications where tumour formation is a concern.
Peace Chukwu
Latest posts by Peace Chukwu (see all)
- New turn for stem cell therapy: How tiny messengers are about to change the game - June 18, 2025
- Menstrual stem cell implication in endometriosis - February 6, 2025
- Menstrual stem cells and their promise - January 10, 2025






Comments