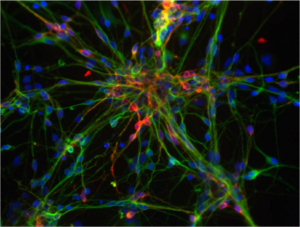
Dompaminergic neurons derived from human embryonic stem cells. These cells degenerate in Parkinson’s disease. This photo was taken by Jeannie Liu in the lab of Jan Nolta at the University of California, Davis. CIRM on Flickr
A recent Wall Street Journal article reminded me of the work of someone I came to know well while at the California Institute for Regenerative Medicine (CIRM): Jeanne Loring of the Scripps Research Institute. The article detailed a cell therapy for Parkinson’s Disease being readied for clinical trials later this year by Aspen Neuroscience, a company that grew out of her lab. The tale of the trek to get to that trial provides a vivid reminder that good science often requires a healthy mix of passion, persistence and attention to detail.
Loring spent the better part of the last two decades working toward that trial, doggedly pursuing new science that might push her team closer to a therapy. At CIRM, our board voted on the recommendations of our grant review committee several times a year and Loring was always there to fight for her grant applications and often with patient advocates beside her. And she did receive nearly US$20 million from CIRM over the years.
Even the patient groups she worked with have gone the extra mile—literally. Instead of a 5K walk through a park, they raised $250,000 by trekking to the top of Africa’s Mount Kilimanjaro in 2011. That group, Summit for Stem Cell Foundation, had raised some $2.5 million for Loring’s work by 2018.
As is often the case with scientists I have come to know well, that passion carries over to personal activities. I interviewed Loring just before she left San Diego for Australia to view the recent total solar eclipse – her 15th.
Much success in regenerative medicine depends on choosing the right cells and the right moment in the cell’s life. Aspen’s trial will use nerve cells created from induced Pluripotent Stem Cells (iPSCs) made from adult cells of specific patients. Loring remains adamant about using these autologous cells and not donor, or allogeneic cells. “People with a debilitating disease should not also be immune suppressed, which is itself a debilitating condition,” she noted. (Read Sara Nolte’s blog about organ and stem cell transplant matching to learn more about this.)
Whenever a research team makes iPSCs, the first major hurdle becomes choosing the right cells growing on a plate. The reprogramming factors that turn adult cells into an embryonic-like state don’t do the job uniformly and only some of the cell colonies become pluripotent. Loring and now Aspen have found ways to use machine learning to choose the best cells. Then as the cells get passaged in culture, it becomes critical to use only cells that haven’t gained damaging mutations as they grow and divide. The team uses an AI approach to spot check the cells while doing whole genome sequencing.
But as you guide the stem cells into the desired cells for transplant, in this case dopamine-making neurons to replace those lost in Parkinson’s disease, at what stage of this differentiation do you transplant the cells? Animal experiments have shown that fully differentiated dopamine neurons don’t implant into the brain well.
“We found a sweet spot in the differentiation where they can fully implant and then differentiate into dopamine producing cells, around five days as defined by gene expression profiles,” Loring said. “At that stage we call them determined.”
Aspen has the precursor neurons for 10 patients frozen and ready when they get the final go ahead from the U.S. Food and Drug Administration to begin the trial. With the automation steps they have developed, it takes just 20 days from the reprogramming stage into iPSCs to the precursors ready to store. This efficient process should eventually bring down the high cost of autologous therapies.
Loring taught embryology for five years. She says an embryologist’s instinct tells her that just as cell-cell interactions guide embryo development, “I fully believe that cell recognition of self-cells will play a role in autologous cell therapy.” But she added, “I can’t prove it yet.”
I am well aware that many in the cell therapy field tout the advantages of the reduced cost of allogeneic (off-the-shelf), donor cells. But Loring and many others stick to a pragmatic fact: allo grafts require immune suppression, which itself can be costly. Those champions of personalized therapies see costs on their side coming down.
Meanwhile, many working on allografts hope to find ways to make the donor cells less likely to trigger dangerous immune responses and reduce the need for immune suppression. That includes two Canadian biotechs that are leaders in the effort.
One firm, panCELLa in Toronto, created what it calls induced Allogeneic Cell Tolerance (iACT) Stealth Cells™ and FailSafe®. The firm engineers iACT Stealth Cells to avoid rejection by the immune system after transplant. FailSafe introduces a suicide gene on the chromosome to control cell proliferation, improving safety for the patient.
London Ontario firm Sernova focuses on improving the quality of life of patients with chronic diseases, specifically diabetes, hemophilia and thyroid disease. They have developed an implantable medical device known as the Cell Pouch System™, which protects therapeutic cells from the patient’s immune system and supports cell function simultaneously. It is organ-like, vascularized and scalable.
I suspect both cell types, allo and auto, will continue to progress toward approved therapies with new clinical trials being announced nearly every month. Our collective knowledge will expand and perhaps we can choose the right cell type for each patient.
Don Gibbons
Latest posts by Don Gibbons (see all)
- Pluripotent stem cell clinical trials for SCI is the target of a “moonshot” consortium - May 28, 2024
- Hope vs hype and champions vs charlatans - August 22, 2023
- One pioneer’s dogged pursuit of personalized cell therapy - May 25, 2023






Comments