I’m excited to be sharing some new Canadian cancer immunotherapy developments! We’ve already seen some great stuff with CAR T cells and checkpoint inhibitors. But the strength of these therapies is also their downfall: their targets make them highly specific and powerful against tumours expressing and exploiting the targets, but also limit the use of the therapies to those specific tumours.
What if rather than making cancer-specific therapies, we made therapy-specific tumours?
That’s exactly what a team led by Drs. John Bell and Jean-Simon Diallo have done. Oh, how the tables have turned!
Using HER2 negative (HER2-) breast cancer as a model, the group from the Ottawa Hospital Research Institute’s Centre for Cancer Therapeutics published their work in Nature Communications this summer. HER2 (human epidermal growth factor receptor 2) is part of a family of proteins extensively involved in cell proliferation and is often over-expressed in breast cancer. Breast cancers that are HER-2 positive are usually treated with the HER2 specific antibody trastuzumab (Herceptin). Briefly, the group used oncolytic viruses (OVs) to simulate HER-2 positivity in previously HER2- breast cancer cell lines, allowing them to become susceptible to the effects of antibody therapy, while simultaneously promoting an endogenous anti-tumour immune response.
Before going into the details of the study (and the really interesting things they’ve done), an overview of oncolytic viruses might be useful. As you may have inferred from the name, oncolytic viruses (OVs) are a group of viruses that preferentially infect and kill cancer cells. Once the cancer cell has been infected, the OV will replicate within the cell until the cell can no longer support replication, at which point the cell lyses (ruptures), releasing hundreds of viral copies. Not only has the OV destroyed a cancer cell, but it has also made more copies of itself to infect more cancer cells. Viral infection and cell lysis also recruit the immune system, which will target infected cancer cells as well.
The next question that comes to your mind is likely: How are OVs tumour-cell specific, especially given that tumour cells are still human cells, and resemble healthy host cells? This can be explained in a few ways:
- To infect a cell, an OV often needs to bind a cell surface protein; tumour cells can have increased, or exclusive, expression of these proteins.
- Viruses require specific internal “machinery” to replicate within a cell; these are also significantly, or exclusively, upregulated in tumour cells.
- Tumour cells have defective DNA repair mechanisms and cell death programming, allowing OVs to replicate fully. A normal cell would likely die before a sufficient viral load could be reached.
Early cancer virology involved using unmodified existing viruses, and testing for their tumour lysing capacity. Now, we can genetically engineer the viruses to improve their efficacy, safety, and/or expand their anti-tumour effects.
This review article provides an excellent summary of the different viruses currently being used as OVs, along with their benefits and limitations.
Now on to the good stuff!
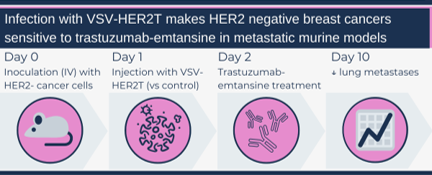
First, the authors engineered a VSV construct that encoded a truncated HER2 protein (HER2T) containing only the cell surface component, but not the internal signalling portion. They not only demonstrated that infection with the VSV-HER2T virus successfully made HER2- cancer cells HER2+, but also that trastuzumab was able to bind these HER2+ cells (both cell lines and patient samples). Similarly, in their metastatic disease mouse model, mice with HER2- cancers injected with VSV-HERT2 and treated with the antibody-chemotherapy combo trastuzumab-emtansine had a reduced burden of disease compared to controls.
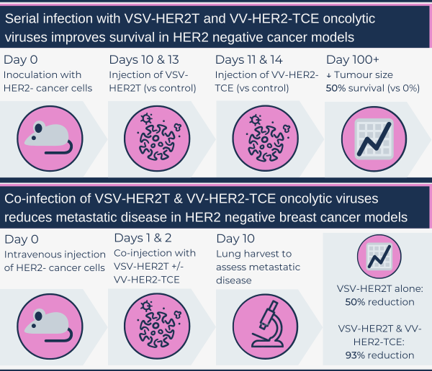
A second OV construct was engineered where a VV strain encoded a HER2-targeted T cell engager (HER2-TCE): The fragment of trastuzumab that binds HER2 was combined with the signalling machinery of CD3, an important co-receptor for T cell binding. The goal of this construct was to amplify a patient’s immune response to HER2+ cells, allowing for T cell-mediated tumour destruction. Mice with HER2+ tumours injected with VV-HER2-TCE demonstrated tumour regression and complete tumour resolution (“cure”) in 40 per cent of the mice. To model disease recurrence, “cured” mice were re-challenged with a repeat tumour cell injection. New tumours were unable to grow, suggesting a preserved anti-tumour immune response.
The really novel approach taken by the team was combining their two OV constructs in the treatment of HER2- cancers. Ex vivo models using human and murine tumour core samples incubated with T cells, with or without OV co-infection, demonstrated more T cell activation with dual infections, compared to single OV or sham controls. When mice with HER2- tumours were treated with various combinations of the OV constructs versus controls, only mice that received both VSV-HER2T and VV-HER2-TCE had a decreased tumour burden and improved survival. Mice with complete tumour regression were challenged with another tumour injection, and tumours were unable to grow, demonstrating a sustained anti-tumour immune response. The authors also tested if the OVs could be administered safely together, which ended up initiating an even stronger intra-tumour T-cell response.
This co-infection strategy was further tested in their advanced metastatic HER2- cancer in vivo models; mice with both OV constructs had the best response (tumour burden reduction and survival), while VSV-HER2T infection alone also had a significant reduction in lung metastases. Co-injection of non-engineered VV and VSV control constructs also led to a 70 per cent reduction in metastases, demonstrating the OVs themselves can generate a significant anti-tumour response.
As highlighted by the study authors in their discussion, the utility of their approach in modifying the tumour cells to express a desired marker allows increased access to existing targeted therapies for more patients. The OV approach also avoids delays associated with CAR T cells – the process involves T cell harvest, in vitro engineering and cell culture, before being re-injected – as the viruses can be prepared in advanced, without production limitations, and injected directly into the patient.
I would have liked to see them include a triple therapy arm that included the dual-virus injection with trastuzumab-emtansine. However, the group rationalizes that the rapidity of the OV-induced tumour cell death would impair the intra-tumour blood supply to the point where any systemically given drugs would have decreased accessibility to the tumour. They further emphasize that their co-administration approach allows for the immediate delivery of the “immunotherapy,” recruiting host T cells before blood supply access is diminished. Based on what we know about cancer, I still think a multi-modal approach would be ideal to test, as relying entirely on the immune system doesn’t seem reasonable either.
The greatest thing about this approach is the promise of reduced systemic disease burden and a sustained immune response that prevents relapse. I would love to see this approach extended to other tumour marker targets (e.g. PD1 in melanoma and metastatic lung cancers). And, of course, I look forward to seeing this novel approach transition into clinical trials.
Sara M. Nolte
Latest posts by Sara M. Nolte (see all)
- Ottawa researchers make tumour-marker negative cancers positive with oncolytic viruses - December 19, 2024
- Cell-based therapy approved as alternative to standard UCB transplant for hematological malignancies - June 21, 2023
- Finding the perfect match: breaking down the science of organ and stem cell transplant matching - April 18, 2023






Comments