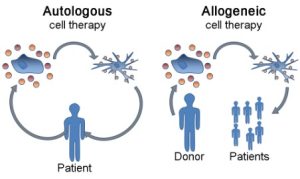
A schematic visualizing the differences between an autologous and allogeneic cell therapy (adapted through a CC license: https://commons.wikimedia.org/wiki/File:Dendritic_cell_therapy_not_annotated.png#filelinks)
Cell therapies that replenish a healthy cell niche have long been an attractive approach to treat a variety of diseases, such as Parkinson’s disease, which are incurable and characterized by the loss of critical cell functions. The attractiveness of cell therapies is not unfounded, as many of these therapies have demonstrated promise in the initial human pilot studies. However, despite the development of these cell therapies dating back over 30 years ago, many regenerative treatments have still not made it to market for one reason or another. Regardless, ongoing advancements in the cell therapy field have continued to keep the dream of curing untreatable diseases alive, as highlighted by Dr. Kevin Robb.
When developing a cell therapy, researchers must decide whether to take an allogeneic or autologous cell therapy approach. Herein, we will discuss the Canadian landscape around allogeneic and autologous therapies, the advantages and disadvantages of each approach, as well as touch on the recent technological advances to allogeneic cell therapies that were discussed at the 2023 Till & McCulloch Meetings (TMM2023), organized by Canada’s Stem Cell Network.
What are autologous and allogeneic cell therapies?
Broadly, autologous cell therapies involve the isolation, processing and re-introduction of an individual’s own cells for the purposes of treating, preventing or curing a disease. There exist several autologous cell therapies approved for use in Canada, with the most notable in recent years being Kymriah (Novartis) and Yescarta (Kite Pharma) — both cell therapies for blood cancers based on chimeric antigen receptor T cells (CAR-T) technology.
In contrast, allogeneic cell therapies isolate and process biological starting material from one donor, and then give the processed product to another individual. Notable allogeneic therapies approved in Canada include cancer treatments, such as hematopoietic stem cell transplants, and Prochymal (Osiris Therapeutics), which is a mesenchymal stem cell-based therapy approved to treat graft-versus-host disease (GVHD). Although it should be noted that Prochymal is not widely used, and ruxolitinib is the only consensus treatment for GVHD. As autologous therapies use an individual’s own biological starting material, there is an assumption that these therapies are safer than an allogeneic alternative.
Current risks associated with autologous cell therapies
However, as summarized in this statement by Health Canada, there are risks associated with autologous cell therapies, which include common surgical risks such as bacterial or viral infections, adverse events arising from cell processing, ectopic tissue or tumour formation, and patient cross-contamination resulting in immunogenic responses. These risks are amplified by the fact that cell therapies can persist in recipients for a much longer period of time than small molecule drugs, greatly increasing the risk of complications. Regardless of the risks, the major factor impeding the development of autologous stem cell-based therapies is the costly logistical issue of keeping track of a patient’s cells to guarantee no cross contamination occurs during the manufacturing pipeline, as such contamination can have profound health and financial consequences. For this reason, there has been a measurable shift by investors towards developing allogeneic cell therapy products, and such products are starting to be approved by the U.S. Food and Drug Administration.
Currently, allogeneic cell therapies have more risk but also have greater economic potential
An off-the-shelf cell therapy to replace or repair damaged tissue is the key driver of allogeneic cell therapies. Being able to engineer a cell therapy from a single donor for therapeutic applications would eliminate the risks of cross contamination, but risks such as those noted above still persist. There is a lot that we do not understand about the human immune system, so developing allogeneic cell therapies, which have the same risk profile as autologous cell therapies, is not yet a reality. In fact, many of these allogeneic therapies require patients to take immunosuppressants, which are very costly.
Advancements to mitigate risks of allogeneic cell therapies

Dr. Akitsu Hotta, Center for iPS Cell Research and Application (CiRA) at Kyoto University (Japan), presented on advancements to mitigate risks of allogeneic cell therapies at the 2023 Till & McCulloch Meetings
At TMM2023, Dr. Akitsu Hotta, assistant professor at Kyoto University, presented their team’s research on methods to reduce the risks of allogeneic cell therapies.
As background, immunosuppression is required for allogeneic therapies because the recipient’s immune system recognizes that the transplanted tissues come from another person. Immune cells, as Dr. Hotta explained, recognize transplanted tissues through the human leukocyte antigen (HLA) system, and thus abolishing the HLA system in the donor tissues (e.g., stem cell-derived transplants) will prevent the body’s immune cells, such as the T cells and natural killer (NK) cells, from being able to detect that the transplanted tissue is from another person.
To abolish the HLA system, many laboratories are developing hypoimmunogenic induced pluripotent stem cells (iPSCs). In particular, Dr. Hotta showed that disrupting the HLA-A, HLA-B and CIITA genes, but retaining HLA-C, was able to circumvent an immunogenic response from T cells and NK cells. Dr. Hotta’s team anticipates that 12 hypoimmunogenic iPSC lines created in this manner could cover 96.4% of the world’s population, greatly reducing the chances that a recipient would reject their allogeneic transplant. It is also possible that allogeneic therapies developed with hypoimmunogenic iPSC lines would also reduce the likelihood of other adverse events like cytokine release syndrome. Importantly, Dr. Hotta has not seen these genetic disruptions to have any impact on the differentiation potential of the iPSC lines, so there is not yet any known limitations of their use (if any). These cell lines are available on the CiRA foundation website for both non-profit and for-profit organizations.
While these advancements are incredibly promising, Dr. Hotta highlighted that there are still several unknowns. First, it is still unclear whether these twelve iPSC lines would truly be rejection-free in real world applications. It is also uncertain whether these genetic disruptions have any impact on tumorigenicity or critical cell functions. Regarding the latter point, there was concern by the conference attendees that disrupting the HLA system could limit the application of these iPSC lines by, for example, preventing their use for hematopoietic cell-based therapies. Regardless, this research certainly will facilitate the progress in developing allogeneic cell therapies and will be particularly beneficial for non-central nervous system (CNS) disorders.
Commenting on immunosuppression requirements of allogeneic cell therapies for CNS disorders
For any disease outside the CNS, there is constant risk of tissue rejection from the immune cells in circulation, and thus immunosuppression is critical. Creating islet cells for patients with diabetes from hypoimmunogenic iPSCs could, for example, mitigate the risk of rejection. In contrast, immune cells in circulation do not readily cross the blood-brain barrier, and thus there is less risk of rejection for CNS transplants, but one still exists!
As explained by Dr. Stefan Irion, the common route of administering a cell therapy into the CNS disrupts the blood-brain barrier, allowing immune cells in circulation to enter the brain parenchyma and attack the tissue transplant. This is the underlying reason why patients in BlueRock Therapeutics’ study received immunosuppressants for a year — to allow the blood-brain barrier to heal. Despite there being some evidence that the native immune cells in the brain, called microglia, can respond to foreign tissue and induce rejection, clinical trials indicate that this may not be something to be widely concerned about, as allogeneic cell therapies have persisted for years in the recipients’ brain parenchyma. However, unexpected life events, such as trauma to the head as well as diseases of the CNS, disrupt the blood-brain barrier. Thus, it is worth noting that hypoimmunogenic iPSC lines may have no perceived benefit in a clinical trial, but they may improve the longevity and safety of cell therapies in the CNS, as this approach may mitigate the risk of transplant rejection later in life due to unexpected life events.
Tyler Wenzel
Latest posts by Tyler Wenzel (see all)
- Promoting cell and gene therapies versus the risks of “scienceploitation” - January 16, 2025
- Casgevy, a world-first CRISPR-based gene therapy, aims to cure sickle cell anemia - December 21, 2023
- Overcoming the limitations of allogeneic transplants to treat incurable diseases, TMM2023 - November 23, 2023






Comments