“Regenerative medicine news under the microscope” is a monthly feature highlighting a selection of impactful research findings and headlines across the many subfields of regenerative medicine.
I’ve gone slightly longer form with this month’s edition, as I found that our coverage of April’s biggest stories would benefit from some additional contextual information. I cover a global assessment of iPSC technologies, iatrogenic Alzheimer’s disease, that spinal cord injury paper, and more.
Pick of the Month:
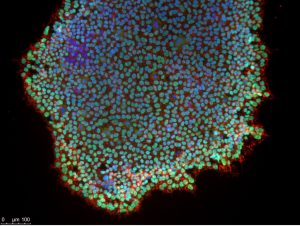
Induced pluripotent stem cells immunostained for pluripotency markers, SSEA4 and Oct4. Nuclei stained with DAPI. Credit: Lyla El-Fayomi.
Great expectations: A global assessment of induced pluripotent stem cell technologies
Induced pluripotent stem cells (iPSCs) are front and centre in regenerative medicine projects around the world. While iPSCs aren’t ready for widespread clinical rollout just yet, they hold much therapeutic promise, with current market value estimates posted at over two billion USD. Lyu et al. aimed to map the global landscape of patents and clinical trials involving iPSCs to track their global development and shed light on current translational challenges. Patent publications have grown rapidly since 2006 (the year Yamanaka’s seminal iPSC paper was published), but that growth has not been reflected in clinical trial trends.
Lyu et al. outline the temporal, geographic and institutional spread of iPSC technologies, even delving into the subfield-specific distribution of these patents. A few notable examples of categories they’ve covered include differentiation (neural, blood, organoids, etc.), application (drug screening, etc.), and gene editing. Trends pertaining to each of these technological groupings are further charted over time; reprogramming, for instance, has understandably been overtaken by differentiation over the years.
In addition to presenting these statistics, the authors discuss how iPSCs are currently being applied and underline areas that show significant potential, balancing this with current barriers in the market. Efficiency and safety issues are also reviewed, touching on concerns such as tumorigenicity and karyotypic abnormalities.
Lots of great information here, don’t miss this read.
A deep dive on iatrogenic Alzheimer’s disease
Bone marrow transplants can benefit patients with certain types of cancer (leukemias, lymphomas, multiple myeloma and some rare solid-tumour cancers), severe aplastic anaemia, immune disorders, and many more; view a full list of the types of cancers here. However, as you can imagine, the genetic quality of a transplanted cell is important. This month, Singh et al. warn that there could be a rare margin for iatrogenic disease in these contexts – that is, disease directly caused by diagnostic or therapeutic interventions.
Animal work in Stem Cell Reports identified a transplantable variant of Alzheimer’s disease (AD), wherein donor bone marrow harbouring a mutated amyloid precursor protein transgene resulted in the development of AD pathologies in transplant recipients. To deal with this newfound risk of genetic contamination, the authors are advocating for genomic sequencing of tissue prior to transplantation; this includes organs, stem cell therapies, blood transfusions and blood-derived products.
An important factor in all this, however, is that the research was preclinical. University College London’s David Curtis, MBBS, MD, PhD, commented on the study for Medscape:
“Theoretically there could be a risk of acquiring Alzheimer’s disease if one received a stem cell transplant from somebody carrying the severe, familial form of the disease. However, this form is extremely rare so in practice the risk seems low and there are many safeguards around stem cell transplantation. I do not see that the risks extend to other areas such as organ transplantation or blood transfusion because these procedures do not involve large numbers of stem cells which can go on to form glial cells.”
See more comments from other experts in the original Medscape piece.
Sadly, real-world examples of iatrogenic AD have been reported before. This past January, Banerjee et al. described a somewhat different situation: They had previously reported on a group of young adults who had been treated in childhood with contaminated, cadaver-sourced pituitary growth hormone. Some of these individuals ultimately died of Creutzfeldt-Jakob disease (CJD), a fatal neurodegenerative disorder. The contamination included two disease-specific proteins: Aβ seeds, implicated in AD, and CJD-causing prions. Their latest work studied survivors of this now-banned procedure who did not die of CJD. Instead, these patients went on to develop dementia and AD-linked pathology as a result of their exposure to Aβ seed contamination.
While these previous findings are a good proof of principle that AD can be acquired from very specific medical procedures under what can be considered rare circumstances, key differences exist across these two cases. Singh et al. worked with genetically diseased donor samples from mice in their Stem Cell Reports paper, as opposed to human samples contaminated with pathological proteins. So, while the outcome was similar across these two papers, the source of the disease differed.
Please note that there is currently no evidence to suggest Alzheimer’s disease is transmissible in day-to-day interactions with patients and loved ones.
A better mesenchymal stem cell for osteoarthritis
Our next story centres around mesenchymal stem cells (MSCs), which have been covered quite extensively here at Signals over the years. These cells show promise in the treatment of osteoarthritis, an incurable degenerative joint disease affecting 15 per cent of individuals aged 65+. Resultant degradation and inflammation of the cartilage can range from mild to severe, causing significant pain and even disability in some patients. More than 200 clinical trials have been registered to study MSC treatment in patients with the condition, but outcomes are not meeting expectations. This month, Chu et al. bring us one step closer to understanding why, citing potential causes as cellular heterogeneity and mechanistic uncertainty. In their paper, they identify a genetically distinct subpopulation of MSCs (marked by Prrx1) derived from white adipose tissue. These distinctive MSCs demonstrate significant multipotentiality and effectively differentiate to replace damaged cartilage in rodents. The authors also shed light on mechanism, confirming the secretion of trophic and anti-inflammatory factors. In their rodent model, these effects lasted at least four months.
The intentional sourcing and “subclassing” of these cells seem to have improved overall results, demonstrating that even samples which might appear similar on the label might not be so.
A positive outlook on stem cell therapies for spinal cord injury
There is very real optimism and some concern over this headline. Dr. Paul Knoepfler, over at The Niche, has already covered it extensively. I’m going to quote him directly before delving into the details:
“If you or a loved one are considering stem cells for paralysis at … a[n unproven] clinic, don’t go that route. There are serious risks including CNS infection. One worry about the over-excited media coverage of this new paper is that clinics will use it for marketing.”
That is to say, these findings are exciting, but still years away from legitimate, widespread adoption by health care providers in non-trial settings.
This month, the Mayo Clinic reported findings from their Phase I trial investigating a treatment for spinal cord injury. Yes, this is another MSC story. Bydon et al. harvested adipose-derived MSCs from patients, expanded them for four weeks, and then injected them directly into injured spinal cords. The study enrolled 10 patients, aged 18 to 65, with 2/10 identified as women, and 3/10 being non-white. The conservative sample size was likely related to the fact that this is a Phase I safety and feasibility trial. A direct quote from the study record on clinicaltrials.gov:
“The purpose of this study is to determine if mesenchymal stem cells (MSC) derived from the fat tissue can be safely administered into the cerebrospinal fluid (CSF) of patients with spinal cord injury.”
I just want to clarify the following for readers who might be less familiar with clinical research: Phase II trials – not Phase I – are where better insight into efficacy and side effects can be gained. See this rough guide by the US Food and Drug Administration (FDA) that explains what each clinical trial phase is best suited for. That’s important to keep in mind when reviewing Phase I data. Still, the results do show potential. Over the course of their 96-week study period, 7/10 patients ultimately demonstrated improvements in motor and/or sensory function. No adverse events were reported, either.
Some additional details that I thought were interesting: Time elapsed between injury and injection fell between seven and 22 months, allowing doctors time to determine whether improvements could be made by way of physical therapy first. Also, the injuries themselves were a mix of cervical and thoracic traumas.
One of the metrics used to assay improvements was the American Spinal Injury Association (ASIA) Impairment Scale, referred to as the AIS score. To learn more about it, this paper is packed full of information including a template of the form used for assessment. In brief, grade A is complete impairment, with no motor or sensory function detected below the injury. At the other end of the spectrum, grade E means a total lack of impairment, with no hindrance to sensory or motor functions detected. While the authors admit this metric has its limitations, their data show that 7/10 patients seem to have graduated at least one letter grade, with some patients even clearing two.
The next phase of studies, which again will be better designed to determine efficacy, should have larger sample sizes and – as both the authors and Dr. Knoepfler have pointed out – controls. However, Bydon et al. do state that patients received the same rehabilitation support as other patients throughout their recovery, pointing to some degree of effort to change as few variables as possible. This makes sense, given that the authors envision their experimental treatment as more of a combination therapy.
Looking forward to the next round of results from this line of investigation.
Additional recommendations
Here are some additional papers and headlines that might interest you:
A simultaneous knockout knockin genome editing strategy in HSPCs potently inhibits CCR5- and CXCR4-tropic HIV-1 infection. Dudek et al. in Cell Stem Cell. Also see the preview by Petronela Ancuta in Cell Stem Cell.
Regeneration of Nonhuman Primate Hearts With Human Induced Pluripotent Stem Cell-Derived Cardiac Spheroids. Kobayashi et al. in Circulation.
3 women contract HIV after ‘vampire facial’ procedures at New Mexico spa. CBC News via The Associated Press. While this is an April 2024 headline, The Niche covered this last summer.
Met investigates ‘stem-cell autism cure’ claim. Jess Warren for BBC News.
Epigenetic mechanisms regulate sex differences in cardiac reparative functions of bone marrow progenitor cells. Thej et al. in npj Regenerative Medicine.
Treating a type 2 diabetic patient with impaired pancreatic islet function by personalized endoderm stem cell-derived islet tissue. Wu et al. in Cell Discovery.
FDA Decision Tracker: Pfizer’s First Gene Therapy Approved for Hemophilia B. Heather McKenzie for BioSpace.
Administration of adipose-derived stem cells extracellular vesicles in a murine model of spinal muscular atrophy: effects of a new potential therapeutic strategy. Virla et al. in Stem Cell Research & Therapy.
Clonal hematopoiesis–derived therapy-related myeloid neoplasms after autologous hematopoietic stem cell transplant for lymphoid and non-lymphoid disorders. Awada et al. in Leukemia.
Trends in Volumes and Survival After Hematopoietic Cell Transplantation in Racial/Ethnic Minorities. Khera et al. in Blood Advances. This was an absolutely massive effort across 41 global affiliations – et al. doesn’t do this justice.
Human anti-PSCA CAR macrophages possess potent antitumor activity against pancreatic cancer. Shah et al. in Cell Stem Cell.
Generation of rat forebrain tissues in mice. Huang et al. in Cell.
Designer peptide–DNA cytoskeletons regulate the function of synthetic cells. Daly et al. in Nature Chemistry.
Micro-patterned culture of iPSC-derived alveolar and airway cells distinguishes SARS-CoV-2 variants. Masui et al. in Stem Cell Reports.
Intron detention tightly regulates the stemness/differentiation switch in the adult neurogenic niche. González-Iglesias et al. in Nature Communications.
Rejuvenating aged stem cells: therapeutic strategies to extend health and lifespan. Matteini et al. in FEBS letters.
The adult environment promotes the transcriptional maturation of human iPSC-derived muscle grafts. Crist et al. in npj Regenerative Medicine.
Genetics of cystogenesis in base-edited human organoids reveal therapeutic strategies for polycystic kidney disease. Vishy et al. in Cell Stem Cell.
FOXO1 enhances CAR T cell stemness, metabolic fitness and efficacy. Chan et al. in Nature. AND FOXO1 is a master regulator of memory programming in CAR T cells. Doan et al. in Nature. Sara Reardon has written a piece about these two highly similar papers for Nature News.
NIBR-LTSi is a selective LATS kinase inhibitor activating YAP signaling and expanding tissue stem cells in vitro and in vivo. Namoto et al. in Cell Stem Cell.
Implantation of a double allogeneic human engineered tissue graft on damaged heart: insights from the PERISCOPE phase I clinical trial. Bayes-Genis et al. in eBioMedicine.
Preclinical efficacy and safety of encapsulated proliferating human hepatocyte organoids in treating liver failure. Yuan et al. in Cell Stem Cell.
Antisense oligonucleotide therapeutic approach for Timothy syndrome. Chen et al. in Nature.
Genetic and functional correction of argininosuccinate lyase deficiency using CRISPR adenine base editors. Jalil et al. in American Journal of Human Genetics.
Craniofacial chondrogenesis in organoids from human stem cell-derived neural crest cells. Foltz et al. in iScience.
Restoration of fertility in nonablated recipient mice after spermatogonial stem cell transplantation. Morimoto et al. in Stem Cell Reports.
Impact of CRISPR/HDR editing versus lentiviral transduction on long-term engraftment and clonal dynamics of HSPCs in rhesus macaques. Lee et al. in Cell Stem Cell.
Recapitulating primary immunodeficiencies with expanded potential stem cells: Proof of concept with STAT1 gain of function. Liu et al. in The Journal of Allergy and Clinical Immunology.
KCNJ2 inhibition mitigates mechanical injury in a human brain organoid model of traumatic brain injury. Lai et al. in Cell Stem Cell.
Reprogramming fibroblast into human iBlastoids. Tan et al. in Nature Protocols.
Apigenin and Rutaecarpine reduce the burden of cellular senescence in bone marrow stromal stem cells. Ali et al. in Frontiers in Endocrinology.
Generation of human alveolar epithelial type I cells from pluripotent stem cells. Burgess et al. in Cell Stem Cell.
Early Data Indicate Cell Therapies Could ‘Reset the Clock’ in Parkinson’s. Patience Asanga for BioSpace.
Parasympathetic neurons derived from human pluripotent stem cells model human diseases and development. Wu et al. in Cell Stem Cell.
Pharmacological expansion of type 2 alveolar epithelial cells promotes regenerative lower airway repair. Shao et al. in PNAS. See also this related coverage on Medscape by Heidi Splete.
Alzheimer’s trial doses patients with stem cell treatment directly to brain. Abigail Beaney for Clinical Trials Arena.
Long-term expandable mouse and human-induced nephron progenitor cells enable kidney organoid maturation and modeling of plasticity and disease. Huang et al. in Cell Stem Cell.
hUC-MSCs therapy for Crohn’s disease: efficacy in TNBS-induced colitis in rats and pilot clinical study. Sun et al. in eBioMedicine.
Mesenchymal stromal cells with chimaeric antigen receptors for enhanced immunosuppression. Sirpilla et al. in Nature Biomedical Engineering.
Structural, angiogenic, and immune responses influencing myocardial regeneration: a glimpse into the crucible. Baccouche et al. in npj Regenerative Medicine.






Comments