“Regenerative medicine news under the microscope” is a monthly feature highlighting big stories in stem cell research. I will sample the latest and greatest findings in recent press and package them into a single post.
August was another packed month in regenerative medicine research news. In this edition, I cover visually-equipped brain organoids, the latest in stem cell therapy for Parkinson’s and Dementia, plus much more.
Also, don’t forget about the new addition to this monthly feature – Pick of the Month! At the end of the year, I’ll remind you of my picks, and we’ll open up a poll to determine your favourite story of the year – Reader’s Pick.
Pick of the Month
When science stares back: 2021’s latest organoids
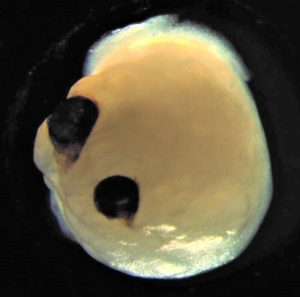
Given my area of study, this story just had to be my pick of the month: brain organoids with impressive, light-sensitive optic vesicles, termed “optic vesicle-containing brain organoids” (OVB-organoids).
Researchers who study the eye can often be overheard saying that a person’s eyes are the only part of the brain you can see without… well, intervening. This is because your retina and optic nerve develop as outgrowths of the brain. In fact, the retina is technically part of your central nervous system.
Framed by this context, OVB-organoids make plenty of sense. By modifying the culture conditions usually employed to grow brain organoids, researchers created an in vitro environment conducive to the continued development and specification of the organoid, allowing for the generation of bilaterally symmetric optic vesicles over the course of 60 days. These extra structures contained functional circuitry and were composed of cells expressing genes related to the developing lens, corneal epithelium, routing of axons at the optic chiasm, the retina, retinal ganglion cells, and more.
One of my favourite parts: The researchers test for light sensitivity using electroretinography, a technique that allows us to record and quantify retinal signaling. When they changed the light intensity, the cells would respond accordingly in a dose-dependent manner.
Future studies might address the outlined limitations of this work, as follows: first, the viability of OVB-organoids past 60 days is apparently questionable, meaning that this model can’t necessarily be used to study mature retinal cells. Also, not all induced pluripotent stem cell donor cells were equally consistent in yielding these organoids following their protocol, so this may be another avenue requiring investigation and optimization.
At this stage, the organoids may be useful in generating retinal pigmented epithelium for transplants by reprogramming patient cells, and may help model the retinopathies associated with early neurodevelopmental disorders.
If, like me, you’re familiar with retinal organoids and are wondering what advantage this system might offer in comparison, here’s the brief version: The optic vesicle-like tissues generated by retinal organoids must be excised and further cultured for a period of several weeks, and thus do not form an in vivo-like structure. You lose the influence of surrounding tissues like the forebrain on the developmental process. Retinal organoids are still remarkable model systems, but each in vitro culture has its pros and cons.
Promising proof of principle prime editing in cystic fibrosis organoid model
When asked about single-gene disorders, most of us tend to think of cystic fibrosis first. It’s incredible to consider how many lives we could save or dramatically improve if we could just edit that one gene. (Learn about cystic fibrosis and exciting Toronto-based research.)
Researchers from the lab of Hans Clevers (yes, the same Hans Clevers behind the crying organoids I highlighted not so long ago) have attempted to use prime editing to correct cystic fibrosis mutations in human stem cell-derived colonic organoids. It’s much safer than conventional CRISPR/Cas9 editing, which makes it a great candidate for application in humans. Whole-genome sequencing was conducted, and no off-target edits were observed, meaning the tool has great specificity. However, editing efficiency did vary, and some undesired mutations did occur at the target site. More optimization is definitely going to be required in the future.
What is prime editing? Well you might recall that I covered base editing in a previous blog; base editing was also an improvement to the original CRISPR/Cas9, as error-prone double-stranded breaks were no longer necessary for editing. A limitation of base editing, however, is that it is limited to “transition” DNA substitutions – meaning an A can be swapped out for a G (and vice versa), or a C for a T, but never an A for a T or a G for a C. Prime editing, on the other hand, can swap out a DNA base for any other, plus it enables the insertion or deletion of up to 80 nucleotides. It has the potential to be an upgrade once optimized, but it works better in certain cell lines over others, and has some way to go. Read a fun take on prime editing from blogger Tara Fernandez.
New in vitro study reveals short-term effects of carbamide peroxide tooth whitening
This is an unusual one relative to what I usually cover, but given how widely used whitening services are, I thought I’d try to raise awareness (and there are stem cells involved, I promise).
The subject of this investigation was carbamide peroxide gel, which is commercially available in the form of at-home whitening kits (if you search these products online, you’ll see concentrations ranging from 5-44 per cent). Why is there interest in this ingredient? After using carbamide peroxide, some people report negative side effects, including tooth sensitivity or burning, irritation, and ulceration of the gums. Researchers wanted to know what could be causing these symptoms.
They measured the effects of varying concentrations of gel on the viability of dental pulp stem cells, gingival fibroblast cells, and the organic content found in enamel. The results were unfortunate: Even concentrations as low as 5 per cent were sufficient to alter the protein content in enamel, increasing enamel permeability and promoting the penetration of extrinsic reagents into the tooth (like peroxide). More specifically, 5-16 per cent concentrations resulted in 50 per cent losses in organic content. With regard to cell viability, 10 and 35 per cent concentrations both acted similarly in reducing cell viability over time, whereas 5 per cent concentrations were safer, affecting viability to a lesser extent.
A critical point here, however, is that because this study was done in vitro, the effects of cellular recovery and any potential host immune response were not tested, so the body’s recovery from the damage was not actually accounted for in these data. The researchers do report previous in vivo findings from the literature, though:
1) Changes in enamel composition can be reversible in vivo; and,
2) with concentrations at 10-16 per cent, reversal of the effect on the gingiva (and therefore, gingival cells) has been reported following a two-week period.
The idea of this is not to fear-monger, but to help us make informed decisions. The authors recommend going for low concentrations of carbamide peroxide, preferably around 5 per cent, to avoid side effects and any potential detrimental effects on overall oral health.
Another step forward in a potential stem cell treatment for Dementia
UCLA researchers have developed a way to grow clinical-grade, transplantable pro-repair astrocytes in large batches.
The group set its sights on brain damage caused by white matter strokes, which can lead to cognitive deterioration progressing for years (a condition called vascular dementia) and even accelerate Alzheimer’s disease. The researchers aimed to promote healing by enlisting the help of cells already known to do so in the brain – asctrocytes. By re-populating these cells in the patient’s brain, the goal was for the transplanted astrocytes to release factors prompting the regrowth and myelination of axons.
Back in April, the same research group showed that this approach could improve both memory and motor ability in a mouse model of the condition, and that the effects were long-lasting. But to bring this therapy to the clinic, they would have to scale up the production of their pro-repair astrocytes in a reproducible and feasible way. This new protocol cut down the required time to produce a transplantable batch of human cells from up to six months, to just 35 days.
The group is aiming to meet with the Food and Drug Administration to advance their research to clinical trials.
Hydrogels for Parkinson’s stem cell transplant therapy
Hydrogels have received plenty of attention in recent years, spanning in utility from tissue repair and drug delivery to contact lens production. In a study by Cameron et al. this month, researchers used hydrogels to help improve the survival and integration of dopaminergic (DA) neuron progenitors to ameliorate the motor deficits seen in animal models of Parkinson’s disease.
Human pluripotent stem cell-derived DA neuron progenitors are subjected to numerous stressors throughout implantation protocols that ultimately affect viability. To improve cell survival, the researchers programmed a biomimetic hydrogel matrix designed to support DA progenitors more effectively throughout the process. Their changes, including the addition of a growth-promoting protein called GDNF, induced a 2.7-fold increase in DA neurons following transplant, in addition to enhanced graft plasticity. This resulted in significant amelioration of motor deficits six months following treatment in animal models.
The authors note that this highlights the benefit of customizing hydrogels so that they’re tissue-specific. They found that it enhanced the functional efficacy of their grafts, and that this result could carry over to other studies as well. In fact, they believe it could be useful in stroke and joint injury models too.
Because the hydrogels are cost-effective and easily manufactured, the next step for this technology would be a clinical trial.
Additional recommendations
Here are some papers/headlines that I didn’t have room for above:
TFEB-mediated endolysosomal activity controls human hematopoietic stem cell fate. García-Prat et al. in Cell Stem Cell.
Hsf1 promotes hematopoietic stem cell fitness and proteostasis in response to ex vivo culture stress and aging. Kruta et al. in Cell Stem Cell.
Generation of pancreatic progenitors from human pluripotent stem cells by small molecules. Jiang et al. in Stem Cell Reports.
U of T’s Medicine by Design helps unite international researchers working to map every human cell. Julie Crljen for UofT News.
Identification of neural oscillations and epileptiform changes in human brain organoids. Samarasinghe et al. in Nature Neuroscience.
Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting. Kang et al. in Nature Communications.
Mesenchymal stem cells and their derived exosomes to combat Covid–19. Dehbidi et al. in Reviews in Medical Virology.
Methacrylic acid-based hydrogels enhance skeletal muscle regeneration after volumetric muscle loss in mice. Carleton et al. in Biomaterials.
Human sensorimotor organoids derived from healthy and amyotrophic lateral sclerosis stem cells form neuromuscular junctions. Pereira et al. in Nature Communications.
Clinics turning to potentially dangerous intranasal stem cell delivery. Paul Knoepfler, The Niche.
APOE4-carrying human astrocytes oversupply cholesterol to promote neuronal lipid raft expansion and Aβ generation. Lee et al. in Stem Cell Reports.
Pluripotent stem cell–derived corneal endothelial cells as an alternative to donor corneal endothelium in keratoplasty. Ali et al. in Stem Cell Reports.
Patient-derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. Lõhmussaar et al. in Cell Stem Cell.
Proliferative stem cells maintain quiescence of their niche by secreting the Activin inhibitor Follistatin. Herrera et al. in Developmental Cell.
Next-generation cancer organoids. LeSavage et al. in Nature Materials.
Adipose micro-grafts enhance tendinopathy healing in ovine model: An in vivo experimental perspective study. Piccionello et al. in STEM CELLS Translational Medicine.
Identification of cancer-related mutations in human pluripotent stem cells using RNA-seq analysis. Lezmi & Benvenisty in Nature Protocols.
Aged skeletal stem cells generate an inflammatory degenerative niche. Ambrosi et al. in Nature.
Stay tuned for my next post, coming up in September!






Comments