“Regenerative medicine news under the microscope” is a monthly feature highlighting big stories in stem cell research. I will sample the latest and greatest findings in recent press and package them into a single post.
Lots to catch up on as we review both December and January’s headlines, so I’ll keep each highlight brief to cover as much ground as possible.
Before we get started, however, your votes have been counted and we have a winner!
Reader’s Choice 2021 goes to…
“Generation of ovarian follicles from mouse pluripotent stem cells” by Yoshino et al. in Science!
Congratulations to this incredibly skilled team, and to all our amazing nominees.
Now, back to our usual business! In this edition, I cover frog leg regeneration, the latest in organoids, a cardiac xenotransplant, and much more!
Pick of the Month(s):
Limb regeneration achieved in the African clawed frog
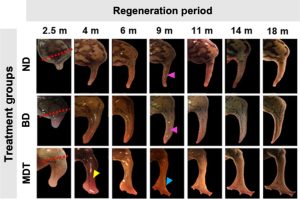
Also known as Xenopus laevis, the African clawed frog has limited regenerative abilities once it reaches adulthood. This quality is shared by humans, which is why X. laevis are a useful animal model in regeneration studies like this one by Morugan et al., out this month in Science Advances.
Following amputation of a hindlimb (conducted under anesthesia and buprenorphine to make this as humane as possible), 24 hours of exposure to a drug cocktail, delivered by a wearable bioreactor, stimulated long-term regrowth. The wearable contained silk protein that was infused with five small-molecule drugs. This regrowth included tissue repatterning, wherein skin, bone, blood vessels and nervous tissue developed. To top it all off, the new hindlimb was functional, allowing the frogs to swim, move and feel.
Clearing senescent cells to improve memory function
This month in Stem Cell Reports, Fatt et al. demonstrate that clearing senescent cells via systemic administration of the senolytic drug Navitoclax results in an acute eruption of neurogenesis – the process by which new neurons are formed in the brain – and this ultimately enhances hippocampus-dependent spatial memory (think: finding your way around a maze). Similar effects were observed when they tried genetically ablating (removal via genetic engineering) senescent cells. In their model, senescent cells in the hippocampal niche adversely affect non-senescent neural progenitor cells during aging. This negative influence is lost when senescent cells are eliminated, resulting in partially restored neurogenesis and thus improved hippocampal function. Don’t miss this read.
Xenotransplantation – pig lends human a heart
What happens to a patient who’s been on cardiac support for almost two months, but whose physiology isn’t compatible with a mechanical heart pump, and who doesn’t qualify for a human transplant due to a history of non-compliance with medical treatment instructions? For 57-year-old David Bennet, all of this meant he would be the recipient of a genetically engineered pig heart. This, of course, required special approval from the Food and Drug Administration – Mr. Bennet’s case was a rare exception. Head over to Nature for a great review of this successful surgical first.
Organoid assembly approach to GI organoids
Gastrointestinal (GI) organs are composed of cells that come from all three primary germ layers of an embryo. The combination of these different lineages lends each organ its trademark complexity. Prior to this month, gastric and esophageal organoids could only be epithelial in composition, meaning the cells only reflect one germ layer, limiting their applications. Setting out to address this using human pluripotent stem cells, Eicher et al. generated GI organoids featuring cells from all three germ layers: enteric neuroglial, epithelial, and mesenchymal precursor cells, each separately derived prior to assembly. The result was a complex gastric organoid highly similar to human stomach tissue, complete with glands and smooth muscle innervated by enteric neurons.
Reprogramming baldness
Antonio Regalado at MIT Technology Review wrote a fun and informative piece highlighting current stem cell-based efforts to find solutions for baldness. He covers a more direct method of re-programming to generate hair stem cells, organoid-based methods of follicle farming, and more. Atop the article lies an unsee-able photo of a hairless little mouse sporting a patch of human hair on the side of its body. Read more here.
Upgrading cortical organoids: unlocking Microglia
We’re hearing more and more about microglia these days; from neurodevelopmental to neurodegenerative disorders, the consequences of their dysfunction can be severe. That being said, prior to this month, a straightforward, reproducible method to generate cortical organoids containing both neurons and microglia had not yet been established. Cakir et al. aimed to change that, successfully using human embryonic stem cells to generate what they call mhCOs – microglia-containing human cortical organoids. To show off their use, the group uses its new mhCO system to model features of Alzheimer’s disease. Though the team highlights a few limitations in their discussion, including that the distribution of microglia within each organoid will vary, this system seems to be the closest we’ve gotten to the mark.
“The first reported evidence of meal-regulated insulin secretion by differentiated stem cells in patients.”
I couldn’t have said it any better. The title above is a direct quote from the paper, which reports on a Canadian clinical trial addressing type I diabetes. While islet cell transplantation is not necessarily new, this procedure has been limited in the past by a shortage of cadaveric (deceased) donors. Given this challenge, Ramzy et al. instead tested a subcutaneous implant containing stem cell-derived pancreatic progenitors in humans. Not only did the cells survive well, but a subset also matured into insulin-secreting cells responsive to glucose in vivo within 26 weeks of implantation. No adverse events were recorded and, overall, patients had 20 per cent lower insulin requirements, saw a 13 per cent improvement in glucose control, and demonstrated enhanced hypoglycemic awareness (the ability to recognize the autonomic symptoms that accompany a drop in glucose levels). These early findings do have their limitations, but substantial progress is clearly being made.
SARS-CoV-2 versus the kidney
Of COVID-19 cases admitted to intensive care, 21.4 per cent have been found to develop stage 2 acute kidney injury (AKI), according to this clinical study. AKI is classified on a scale of 1 to 3, with three being the most severe. Chronic kidney disease has also been identified in “long COVID” patients. To figure out whether or not SARS-CoV-2 could damage the kidney via direct infection, Jansen et al. exposed human kidney organoids to the virus. The quick answer is yes, direct infection is indeed possible, and it definitely causes damage – adding to the long list of organs that SARS-CoV-2 is capable of attacking.
LentiGlobin update: The experimental gene therapy for sickle cell
An experimental gene therapy developed for sickle cell disease has been found to eliminate the most severe complication associated with the condition, in a new study by Kanter et al. In patients with sickle cell disease, a mutated beta-globin gene results in abnormally-shaped hemoglobin. This abnormal shape leaves red blood cells prone to clumping together, and these clumps can block blood vessels and damage organs. LentiGlobin therapy involves the harvesting of blood-forming stem cells from a patient (35, in this study), infecting these stem cells with a lentivirus carrying a healthy beta-globin gene, and later reinfusion of the stem cells (which now carry the non-diseased beta-globin gene) back into the patient. Fantastically, the authors observed complete resolution of severe vaso-occlusive events during the study period.
A critical limitation of this therapy was that untreated stem cells in the patient (the ones carrying the disease gene) were eliminated using chemotherapy prior to the transplant, and this approach was linked to two cases of leukemia. The authors have acknowledged that they will have to find safer alternatives to chemo in future work.
Fallopian tube organoids to study ovarian carcinogenesis
Last month in Cell Reports, Yucer et al. outlined their human induced pluripotent stem cell (iPSC)-derived fallopian tube organoids. They used patient iPSC lines with specific mutations in the BRCA1 gene linked with ovarian cancer and differentiated the cells to become fallopian tube epithelium. These new organoids display cancer phenotypes, plus re-capitulate the severity of the cancer found in the original patient (the cell line donor). The authors suggest that these organoids may be useful both to predict cancer severity, and as a drug screening platform.
Additional recommendations
Here are some papers/headlines that I didn’t have room for above:
DECEMBER
Heart neurons use clock genes to control myocyte proliferation. Tampakakis et al. in Science Advances.
How Canadian researchers are using stem cell advances to transform medicine. Dene Moore for The Globe and Mail.
Co-emergence of cardiac and gut tissues promotes cardiomyocyte maturation within human iPSC-derived organoids. Silva et al. in Cell Stem Cell.
Epithelial phenotype restoring drugs suppress macular degeneration phenotypes in an iPSC model. Sharma et al. in Nature Communications.
Mesenchymal stromal cells mitigate liver damage after extended resection in the pig by modulating thrombospondin-1/TGF-β. Nickel et al. in npj Regenerative Medicine.
Profiling senescent cells in human brains reveals neurons with CDKN2D/p19 and tau neuropathology. Dehkordi et al. in Nature Aging.
Researchers working on injection-free cell therapy for diabetes. Julie Crljen for UofT News.
This Startup Is Making—and Programming—Human Cells. Matt Reynolds for Wired.
Human blastoids model blastocyst development and implantation. Kagawa et al. in Nature.
A genetic modification that reduces ON-bipolar cells in hESC-derived retinas enhances functional integration after transplantation. Yamasaki et al. in iScience.
A bioinspired gelatin-hyaluronic acid-based hybrid interpenetrating network for the enhancement of retinal ganglion cells replacement therapy. Dromel et al. in npj Rejenerative Medicine.
Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles. Sahu et al. in Nature Aging.
Single-cell delineation of lineage and genetic identity in the mouse brain. Bandler et al. in Nature.
Pluripotent stem cells related to embryonic disc exhibit common self-renewal requirements in diverse livestock species. Kinoshita et al. in Development.
JANUARY
Urgent appeal to the Government of Ontario – Now is the time to support regenerative medicine. Ontario Institute for Regenerative Medicine (OIRM).
When stem cells meet COVID-19: recent advances, challenges and future perspectives. Li et al. in Stem Cell Research & Therapy.
Esophageal regeneration following surgical implantation of a tissue engineered esophageal implant in a pediatric model. Sundaram et al. in npj Regenerative Medicine.
CRISPR activation enables high-fidelity reprogramming into human pluripotent stem cells. Sokka et al. in Stem Cell Reports.
Joint-on-chip platforms: entering a new era of in vitro models for arthritis. Paggi et al. in Nature Reviews Rheumatology.
An immunologically active, adipose-derived extracellular matrix biomaterial for soft tissue reconstruction: concept to clinical trial. Anderson et al. in npj Regenerative Medicine.
Lipid droplet dynamics regulate adult muscle stem cell fate. Yue et al. in Cell Reports.
In vitro disease modeling of oculocutaneous albinism type 1 and 2 using human induced pluripotent stem cell-derived retinal pigment epithelium. George et al. in Stem Cell Reports.
Biomanufacturing in low Earth orbit for regenerative medicine. Sharma et al. in Stem Cell Reports.
Epicardial slices: an innovative 3D organotypic model to study epicardial cell physiology and activation. Maselli et al. in npj Regenerative Medicine.
Lentiviral globin gene therapy with reduced-intensity conditioning in adults with β-thalassemia: a phase 1 trial. Boulad et al. in Nature Medicine.
Down-syndrome-induced senescence disrupts the nuclear architecture of neural progenitors. Meharena et al. in Cell Stem Cell.
Keio Univ. team from Japan uses iPS-derived cells to treat spine injury in world 1st. Suzuko Araki for The Mainichi.
SARS-CoV-2 Viral Genes Compromise Survival and Functions of Human Pluripotent Stem Cell-derived Cardiomyocytes via Reducing Cellular ATP Level. Liu et al. on bioRxiv. Note this has not yet been peer-reviewed.
Robust differentiation of human enteroendocrine cells from intestinal stem cells. Zeve et al. in Nature Communications.
Stay tuned for my next post!






Comments