Regenerative Medicine News Under the Microscope is back! Thank you all for your patience as I wrapped up my PhD; the hiatus is officially over. We’re celebrating the return of this feature with a double header covering regenerative medicine research highlights from both February and March.
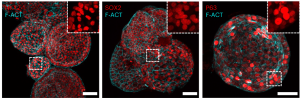
Representative amniotic fluid-derived lung organoids. Cells are immunofluorescently stained for lung-specific stem and progenitor markers. From Gerli et al. in Nature Medicine.
Pick of the month(s):
Human amniotic fluid gives rise to fetal lung, intestinal, and kidney organoids
Congenital malformations affect between 3-6 per cent of babies around the world. For the first time, organoids grown from primary human epithelial progenitors found in amniotic fluid could provide insights into both the root cause and the progression of these malformations. This discovery by Gerli et al. serves as a significant advancement in personalized prenatal medicine; samples can now be obtained from ongoing pregnancies to gauge the efficacy of specific treatments, yielding results in just the 4-6 weeks required to grow the organoids. These tissues were previously inaccessible to medical teams in the context of treating an actively developing fetus; in the past, only terminated pregnancies could be used for this type of research, resulting in both legal and ethical challenges. Unfortunately, such efforts would primarily serve future patients as opposed to facilitating and informing present medical interventions, highlighting the potential inherent in this new approach taken by Gertli et al.
Guided by single-cell sequencing, the authors generated a human amniotic fluid cell atlas and used it to identify and isolate lung, gastrointestinal and renal progenitor cells. This allowed them to culture what they’ve termed amniotic fluid organoids (AFOs) that model fetal lung tissue, kidney tubules and the small intestine. As a proof of concept, lung AFOs were grown from the amniotic fluid of fetuses with a condition called congenital diaphragmatic hernia (CDH), afflicting one in 3000 births. CDH causes a hole to be formed in the diaphragm, leaving nothing to prevent the bowels, stomach or liver from migrating into the chest cavity. This in turn limits the amount of space available for the respiratory system, often resulting in serious and life-threatening complications such as pulmonary hypoplasia: underdevelopment of the lungs.
Lung AFOs were collected from fetuses before and after treatment for the disease, which is called fetoscopic endoluminal tracheal occlusion (FETO): the insertion of a small balloon into the trachea to trap lung fluid inside the airway, stretching it to markedly improve growth of the lungs themselves. See a useful paper on this here. Growth improvements induced by FETO manifested in the organoids grown from amniotic fluid, demonstrating significant differences in development as a readout of treatment success. Dr. Paolo De Coppi captured it best in his quote for the Guardian: “This is the first time that we’ve been able to make a functional assessment of a child’s congenital condition before birth.”
Future work could provide insights into other congenital conditions, such as cystic fibrosis. There are also hopes that AFOs can be used in drug testing. To read more about the details and possibilities, bookmark this research briefing at Nature, or head to the original work here.
Vitamin A at the intersection of hair growth, wound healing and cancer
Epidermal repair involves more than just skin stem cells. Hair follicle stem cells, which are usually responsible for hair growth, can also get involved to support healing under urgent circumstances. To do so, they enter a state of lineage plasticity where transcription factors corresponding to both stem cell types (skin and hair) are expressed. Until the cell’s fate is ultimately decided, though, it can’t function properly in either domain. Enter retinoic acid (RA), a metabolite of vitamin A, discovered by Tierney et al. to be critically involved in the regulation of said lineage plasticity and differentiation. They found that RA levels had to fall for hair follicle stem cells to become engaged in wound repair, as high levels do not allow for this. However, if RA levels dip too low, hair follicle stem cells divert excessive effort towards wound repair at the expense of hair growth. That’s why it’s important that once healing is complete, RA levels rise again to allow for hair regrowth. This dose response curve may also shed light on cancer biology: if the suppression of lineage plasticity is found to be useful in controlling the growth of tumours, retinoids may help improve outcomes. Read more on this work and its implications at Rockefeller University’s Science News.
A hydrogel escort for iPSCs treating Parkinsonian rats
Hydrogels have been making waves in the regenerative medicine field for quite a while now. They are biomaterials that have been found to boost the success rates of certain stem cell transplants by improving cell adhesion, providing neurotrophic factors that promote survival and differentiation, plus lending protection from the host’s immune system. If you’re not already familiar with them, consider this technical review in Molecules, or this one in Gels. There’s been a lot of local hydrogel buzz here in Toronto as well, a notable example being Dr. Molly Shoichet’s work at the University of Toronto. See these two reviews from her lab, here and here.
Hydrogels are being used to aid in regeneration of many different tissue types, but this highlight specifically covers efforts to deliver healthy dopaminergic neurons to patients with Parkinson’s disease. Previous work in Scientific Reports and the European Journal of Neuroscience demonstrated the feasibility of this idea using fetal-derived dopamine neurons, where the hydrogel was found to improve both survival and the capacity of these cells to reinnervate the brain, leading to a significant improvement in motor function. However, work published this past month has specifically adapted this protocol using human induced pluripotent stem cells (iPSCs) instead of fetus-derived cells. iPSCs hold much promise for this type of therapy in the clinic (as is the case in this Kyoto Trial: UMIN000033564), so Comini et al.’s new data offer greater clinical relevance overall. They recorded an 8-fold improvement in transplanted cell survival, and a 16-fold enhancement in differentiation to the desired dopaminergic fate, so their results were positive. Read more in a release from the University of Galway here.
Bone marrow-derived mesenchymal stem cells take on inflammatory bowel disease
The incidence of inflammatory bowel disease (IBD) is increasing every year. The current estimates are that more than 7 million people in the U.S. and Europe will have IBD by the year 2030. Characterized by symptoms including abdominal pain, bleeding and weight loss, this disease seriously and adversely impacts one’s quality of life. It seems to be a symptom of industrialization, with newly industrialized countries experiencing a rapid increase in cases.
New work out in Scientific Reports has shown that mesenchymal stem cells derived from bone marrow, through their anti-inflammatory and antioxidant properties, may be a promising future therapeutic option. Following two weeks of treatment in a mouse model of ulcerative colitis (a type of IBD), genes related to inflammation were downregulated. In addition, there was a protective effect on the enteric nervous system, meaning a reprieve for neurons that innervate the gastrointestinal tract and that are usually damaged by oxidative stress in chronic colitis. Given that treatment options for IBD aren’t very effective, and many refractory cases end in surgical resection of the diseased bowel, these findings are welcome news.
Also, if you’re interested in IBD and its links to environmental factors, the GIVES-21 consortium is currently investigating IBD epidemiology with a specific focus on industrialization in Asia, Africa and Latin America. This Nature Perspectives piece is also informative.
Man remains in remission from HIV-1 five years after hematopoietic stem cell transplant
This headline has received quite a bit of media attention already, so I’ll be brief. Mr. Paul Edmonds, a 68-year-old man who lived with HIV-1 for 31 years, was cured of both HIV and leukemia thanks to a single treatment. (Keep in mind, he did undergo chemotherapy at reduced intensity as well, which was prior to the stem cell transplant.) He is only the fifth person in the world to have escaped both illnesses. A correspondence piece in the New England Journal of Medicine this February revealed that Mr. Edmonds received a hematopoietic stem cell transplant for acute myeloid leukemia 60 months ago, back in 2019. He remains free of HIV-1 today and has not needed to take antiretroviral therapy for about three years. The key to his success appears to be transplant cells sourced from a donor harbouring a mutation linked with HIV-1 immunity: CCR5-Δ32/Δ32. Current estimates show that only 2 per cent of the population has this mutation. If you want to read more, check out this article in The Guardian. This one from ABC News is also very useful, including words of caution from Dr. Anthony Fauci on the matter.
Additional recommendations
Here are some papers/headlines that I didn’t have room for above:
APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia. Haney et al. in Nature.
In ‘major milestone,’ FDA approves first cell therapy for solid tumors. Angus Chen for STAT News.
Alzheimer’s Transmissible Via Stem Cell Transplantation? Megan Brooks for Medscape.
Adipose mesenchymal stem cell-derived exosomes promote skin wound healing in diabetic mice by regulating epidermal autophagy. Ren et al. in Burns & Trauma.
Exonic knockout and knockin gene editing in hematopoietic stem and progenitor cells rescues RAG1 immunodeficiency. Castiello et al. in Science Translational Medicine.
Novel stem-cell therapy continues to show promise for Parkinson’s disease. UCI Health.
Harvard longevity scientist sparks furor with claim about reversing aging in dogs. Megan Molteni for STAT news; and the preprint stirring up all the controversy, A Randomized, Controlled Clinical Trial Demonstrates Improved Cognitive Function in Senior Dogs Supplemented with a Senolytic and NAD+ Precursor Combination. Simon et al. in BioRxiv.
A micro-fragmented collagen gel as a cell-assembling platform for critical limb ischemia repair. Chung et al. in Bioactive Materials.
Signal of Benefit for Stem Cell Therapy in Progressive MS. Ted Bosworth for Medscape.
Embryonic stem cell transplant showing safety in 12 patients. Lindsey Shapiro for Parkinson’s News Today.
Inflammation-suppressing cornea-in-a-syringe with anti-viral GF19 peptide promotes regeneration in HSV-1 infected rabbit corneas. Simoliunas et al. in npj Regenerative Medicine.
Reconstructed Human Skin with Hypodermis Shows Essential Role of Adipose Tissue in Skin Metabolism. Jäger et al. in Tissue Engineering and Regenerative Medicine.
Naturally occurring T cell mutations enhance engineered T cell therapies. Garcia et al. in Nature.
Generation of complex bone marrow organoids from human induced pluripotent stem cells. Frenz-Wiessner et al. in Nature Methods.
A versatile CRISPR-Cas13d platform for multiplexed transcriptomic regulation and metabolic engineering in primary human T cells. Tieu et al. in Cell.
Stealthy stem cells to treat disease. Elie Dolgin for Nature.
Engineering human pluripotent stem cell lines to evade xenogeneic transplantation barriers. Pizzato et al. in Stem Cell Reports.
Label-Free and High-Throughput Removal of Residual Undifferentiated Cells From iPSC-Derived Spinal Cord Progenitor Cells. Nguyen et al. in Stem Cells Translational Medicine.
Autologous transplantation of P63+ lung progenitor cells for chronic obstructive pulmonary disease therapy. Wang et al. in Science Translational Medicine.
Cerebrospinal fluid mtDNA concentrations are increased in multiple sclerosis and were normalized after intervention with autologous hematopoietic stem cell transplantation. Pavlovic et al. in Multiple Sclerosis and Related Disorders.
Autologous hematopoietic stem cell transplantation for multiple sclerosis: Long-term follow-up data from Norway. Kvistad et al. in Multiple Sclerosis Journal.
Multipotent bone marrow cell–seeded polymeric composites drive long-term, definitive urinary bladder tissue regeneration. Bury et al. in PNAS Nexus.
ER stress and lipid imbalance drive diabetic embryonic cardiomyopathy in an organoid model of human heart development. Kostina et al. in Stem Cell Reports.
Human iPSC-derived photoreceptor transplantation in the cone dominant 13-lined ground squirrel. Yu et al.in Stem Cell Reports.
Human-induced pluripotent stem cell-derived neural stem/progenitor cell ex vivo gene therapy with synaptic organizer CPTX for spinal cord injury. Saijo et al. in Stem Cell Reports.
MSX1+PDGFRAlow limb mesenchyme-like cells as an efficient stem cell source for human cartilage regeneration. Liao et al. in Stem Cell Reports.
Restoration of fertility in nonablated recipient mice after spermatogonial stem cell transplantation. Morimoto et al. in Stem Cell Reports.
IL-24 improves efficacy of CAR-T cell therapy by targeting stemness of tumor cells. Zhang et al. in British Journal of Cancer.
X-irradiated umbilical cord blood cells retain their regenerative effect in experimental stroke. Yasui et al. in Scientific Reports.
The Donation of Human Biological Material for Brain Organoid Research: The Problems of Consciousness and Consent. Kataoka et al. in Science and Engineering Ethics.






Comments