The 10 percent rule usually works fairly well when estimating disease prevalence in Canada compared to the equivalent in the U.S. Our population is about 10 percent the size of the U.S. so it makes sense that we match up, to an equivalent degree, on health issues, with some exceptions.
Now, it seems, we are living up to expectations when it comes to the number of unapproved stem cell clinics we have in Canada.
Leigh Turner, one half of the team to catalogue the 570 clinics in the U.S. offering direct-to-consumer marketing of stem cell treatments that were not compliant with the Food and Drug Administration, has now done the same for Canada. His study found 43 Canadian clinics marketing stem cell products that have not been approved by Health Canada and concludes, alarmingly, that “the Canadian direct-to-consumer marketplace for stem cell interventions appears poised for expansion.”
(By the way, in the same study about Canadian clinics, Dr. Turner writes that a May 2017 follow-up study on U.S. clinics “selling unproved stem cell interventions” found 716.)
Patients willing to take a chance on these clinics and unwilling to wait for Health Canada approval may argue that since it’s their bodies and they are paying out of pocket (i.e. not taxpayer funded as provincial insurers do not currently reimburse for these treatments), what is the harm apart from the money they’ve wasted if the treatment doesn’t work? Their choices don’t impact society as a whole.
As the International Society for Stem Cell Research points out, there is more to consider. Such as:
- Complications may create new short- and long-term health problems, and/or may make your condition or symptoms more difficult to manage.
- Receipt of one unproven or experimental treatment may make you ineligible for future clinical trials or treatment options.
(For the full list of considerations, read Nine Things To Know About Stem Cell Treatments.)
In any case, the harm does go beyond the personal and has consequences for society.
There is so much hope for stem cell treatments that the hype remains high. (That’s how these businesses are able to attract clients.) Unfortunately, as Dr. Turner points out in this interview with RegMedNet, “there is the confusion and misunderstanding that exists as a result of companies blurring distinctions between established treatments and unproven, experimental interventions. […] I’m very concerned about the ethical and legal issues generated by such commercial and clinical activity as well as their implications for health policy and health care systems.”
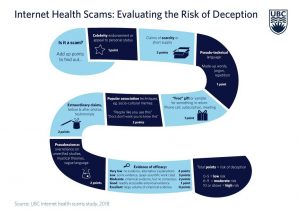
Perhaps this fun and easy to use “Risk of Deception Tool,” produced by a team at the University of British Columbia, will help prospective patients evaluate the promises made by Canadian clinics offering stem cell interventions. Don’t let that backwards “s” shape signify “sucker.”
Stacey Johnson
Latest posts by Stacey Johnson (see all)
- Right Turn: Top 10 blogs from 2025 - January 9, 2026
- Right Turn: Season’s greetings and upcoming event - December 25, 2025
- Right Turn: Stem cell supplements: A growing market with growing risks - December 19, 2025






Comments