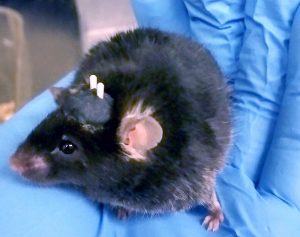
The author’s mouse following preparation for in vivo optogenetics experiments. She performed stereotaxic neurosurgery to a) virally deliver the opsin gene to the brain region of interest through targeted, bilateral injections, and b) implant fibre optic cables hovering just above her region of interest to guide light into the tissue (white horn-looking structures on the mouse’s head, secured using black dental acrylic).
A mouse with mysterious cables attached to its head explores an enclosure, casually walking past a small wooden stick, uninterested.
Cut to another scene of the same mouse as it walks past the same stick – only this time, a light atop its head begins to glow. Without hesitation the mouse pounces, grabbing the stick and gnawing at it violently.
What I’ve just described to you is research published in 2017 by Araujo et al, suggesting that the central amygdala is the seat of predatory hunting behaviour – and they induced that behaviour by activating neurons with light.
If I were asked to summarize optogenetics in a few words, I would describe it as a genetic engineering strategy that allows scientists to manipulate cell physiology using light of a particular wavelength. Although it has largely been regarded as a tool used exclusively for neuroscience applications both in vivo and in vitro, many tools are being developed to control the physiology of non-neural cell types, including stem cells.
Humble Beginnings
Our story begins with the green algae Chlamydomonas reinhardtii, a motile, unicellular organism that photosynthesizes to survive. What’s fascinating about these tiny eukaryotes is that they’ve got an organelle called an “eyespot” that allows them to detect light from the sun and then swim towards it to make food.
Within the eyespot is an ion channel called channelrhodopsin-2. It is bound to a molecule called all-trans retinal, which is the source of its light-sensing capabilities. When blue light – and only blue light – hits all-trans retinal, it forms a kink at the end of its molecular chain to become 13-cis retinal, changing the conformation of channelrhodopsin so that it opens and allows sodium ions into the cell.
You may already recognize this as a familiar phenomenon if you’ve ever been interested in neuroscience. Neuron action potentials, defined as the electrical activation of a brain cell, are produced by a rapid flux of sodium streaming into the cell.
Logistically, it was thought that attempts to achieve control over brain cell activity by genetically engineering neurons to express channelrhodopsin-2 would be ineffective, and even toxic. However, in 2005, this feat of neuroscience was completed for the first time with enormous success by Drs. Edward Boyden and Karl Deisseroth, meaning that neuroscientists had finally gained the ability to control action potentials with precision in the domain of milliseconds.
Why was this discovery so important? Well, up to this point, researchers had been using chemicals, electrical stimulation, and genetic knock-outs to study different parts of the brain. Now, neuroscientists studying conditions such as addiction, Parkinson’s, or depression (to name just a few) can activate neurons with far greater specificity to better understand healthy versus unhealthy circuits. Beyond this, the optogenetic toolkit has expanded into non-neuroscience fields, enabling biologists to study molecular events including gene expression and protein interactions at tightly-controlled timescales.
Below, I explain optogenetic tools in greater detail – both for general cell biology and neuroscience applications – and follow up with some resources that I’ve found to be incredibly helpful. (Ed: Lyla is about to get more technical, for those of you wanting to exit here.)
Unprecedented Precision
Optogenetic tools offer unprecedented, multi-layered spatial and temporal specificity. The first layer of spatial specificity comes from the fact that you can guide light into very specific parts of the brain using a special fibre optic implant, or an LED array for cell culture applications. Perhaps more significantly, you can drive the expression of channelrhodopsin-2 using cell type-specific gene promoters. Even if you’re targeting a brain region or culture dish containing heterogeneous cell types, you can select just one and activate it exclusively.
One example of a technique used to gain this type of specificity in vivo is by stereotaxically injecting an adeno-associated virus carrying a floxed opsin gene into the brain of a mouse genetically engineered to express a Cre recombinase. Expression of the Cre can be driven by, for example, a GABA-specific promoter. Such a system will ensure that you will only have expression of the opsin in GABA+ cells, because only they express the Cre recombinase required to orient the opsin gene correctly.
Temporal specificity, on the other hand, comes from the fact that the effects of activation can be seen almost instantly upon light initiation, and activation is reversible. The second layer is control over the temporal arrangement of action potential firing over time.
The combination of spatial and temporal control is not offered by typical techniques in the field, such as those involving chemical agonists or antagonists, and electrical stimulation.
Channelrhodopsin-2 is but the tip of the iceberg: there have been a slew of variants discovered since then; some produced in a lab, and some found in nature. There are now opsins that can inhibit neurons upon light activation, follow light pulses at up to 200 Hz per second (in contrast to channelrhodopsin-2, which will follow a maximum of 40 pulses per second), opsins that function similarly but that respond to different wavelengths of light, and much much more. Choosing which opsin is best for your neuroscience experiments requires a combination of considerations surrounding its effect on membrane potential, channel or pump kinetics and activation spectrum.
Not Just for Neuroscientists
Now, what if you wanted to investigate cell signalling, protein-protein interactions, or differentiation?
“Cellular optogenetics” has been used to study all of the above, as some proteins can be engineered to contain light-sensitive domains. A review by Drs. Heath Johnson and Jared Toettcher, published last year, summarized the use of such tools for the study of developmental biology in wonderful detail. Potential applications include the selective illumination of cells during specific temporal windows, or on very limited areas of a tissue, to determine when and where the expression of a gene or a signalling event is most important during the process of development. However, they emphasize that although it is still early days in the field, and there is still much to do in the way of tool development, the “first wave” of work has laid a promising foundation.
In their review, they highlight a recent study wherein researchers were able to partially control vulval fate in c. elegans by optically manipulating the nuclear import of lin-1. They also discuss specific examples of optogenetic control over receptors: receptor tyrosine kinases have been manipulated in mammalian cells and, in zebrafish, the role of TGF-β receptor signaling in the process of embryogenesis has also been explored using light.
Other notable examples of stem cell studies employing optogenetics include a study published by researchers at UC San Francisco. Optogenetics was used to drive the differentiation of mouse embryonic stem cells to neurons by manipulating the expression of Brn2 using light. As a result of the temporal precision offered by optogenetics, they also revealed a timing mechanism used by stem cells to decide whether a signal is simply noise, or if it’s important enough to pay attention to. By experimenting with the length and strength of light pulses, they discovered that an expression threshold must be reached before the cell responds to the signal and differentiates.
The last study I’ll mention was a landmark paper by Imayoshi et al. They demonstrated that distinct cell fates can be induced in neural progenitor cells depending on whether the expression of transcription factor Ascl1 is oscillatory versus constant. Oscillatory expression maintained multipotency, while consistent expression promoted differentiation to neurons.
Optogenetics image courtesy of Dr. Ed Boyden’s lab
Getting Started with Optogenetics
If you are considering beginning a new research project using optogenetics, you may already know that there are many experimental parameters and potential confounds that must be controlled for. This includes, but is not limited to:
- a) Selecting the right optogenetic tool for the physiology of your cell and intended activity induction, paying special attention to protein kinetics;
- b) Maintaining an awareness of any potential caveats associated with your selected tool;
- c) Knowing the effects of light wavelength, intensity, pulse duration and pulse frequency on both your cell of interest and selected tool;
- d) Gene delivery method;
- e) Controlling for potential off-target stimulation; and,
- f) The strategies that you plan on using to validate proper expression of the light-sensitive protein and induction of only the intended cell activity.
The fact that this list is not necessarily comprehensive says a lot about the complexity of optogenetic tools; however, when designed the right way, optogenetics experiments can provide marvellous insights into animal behaviour and physiology.
Before getting started, perhaps invest some time in the Optogenetics Training Series created by the Society for Neuroscience, which launched last year. As a graduate student using optogenetics for behavioural neuroscience research, I have found it to be extremely valuable; in fact, points a) through f) above were obtained from their series. They provide an extensive primer on optogenetics both in vivo and in vitro, and provide you with expertise from a wide array of experts across multiple training modules. Other excellent resources include the laboratory websites of Drs. Boyden and Deisseroth.
If you are interested in working in vivo, surgical skills will be required. The Journal of Visualized Experiments has published a helpful video by researchers at Baylor College of Medicine demonstrating everything from implant construction to the surgery itself. I do this surgery as part of my research, and I whole-heartedly recommend learning it first-hand from someone with experience – especially if you’re working with mice, which are smaller and more delicate. Observing and being observed will make a substantial difference in the quality of your work.
For those considering optogenetic work to examine signaling or protein interactions, another review by Dr. Toettcher and Alexander Goglia offers an in-depth and up-to-date report on the current state of the cellular optogenetic toolkit.
I hope that you find these resources as helpful as I did. Whether you intend to bring optogenetics into your research, or you just plan on keeping up to date with the latest in light, I wish you the very best of luck with your endeavours! I can’t wait to see where this field takes us.






Comments