Cancer immunotherapy is so hot right now. Signals bloggers have covered CAR T cells (here and here), but haven’t given some other exciting immunotherapies their time to shine. I aim to correct that.
A normal part of the immune system, checkpoints are a mechanism to prevent the immune response from running rampant and destroying host cells. Some cancers have developed ways to evade the immune system by promoting checkpoint signalling. Hence, the development of checkpoint inhibitors (CPIs): a class of immunotherapies that work to supress the “off” signals put out by a tumour, allowing the immune system to activate and mount an anti-tumour immune response.
However, in some cancers (e.g. melanoma and certain breast cancer subtypes) CPIs are not as effective. These tumours are considered to be immunologically “cold.” This means that although the “off” switch is suppressed, there is still little to no activation of the immune system: it requires an additional “on” switch.
With this dilemma in mind, researchers at the Pritzker School of Molecular Engineering (University of Chicago), have set out to find a way to make these “cold” tumours “hot.”
In April 2020, Drs. Jeffrey Hubbell and Melody Swartz published a very promising solution in Nature Biomedical Engineering: fusing interleukin-12 with another protein’s collagen-binding domain. (An improvement on last year’s strategy, which showed unsatisfactory results in aggressive tumours.)
What makes this fusion protein so effective are its two components:
- Interleukin-12 (IL-12): a very powerful activator of the immune system, signalling for and perpetuating the activation of T cells (immune system “soldiers”);
- The collagen-binding domain (CBD): borrowed from von Willebrand factor; binds the exposed collagen (structural protein) of the “leaky” blood vessels comprising the tumour vasculature.
The fusion protein CBD-IL-12 is able to bind and accumulate within the tumour due to significant amounts of exposed collagen, thereby turning the “cold” tumour into an immunological hotbed.
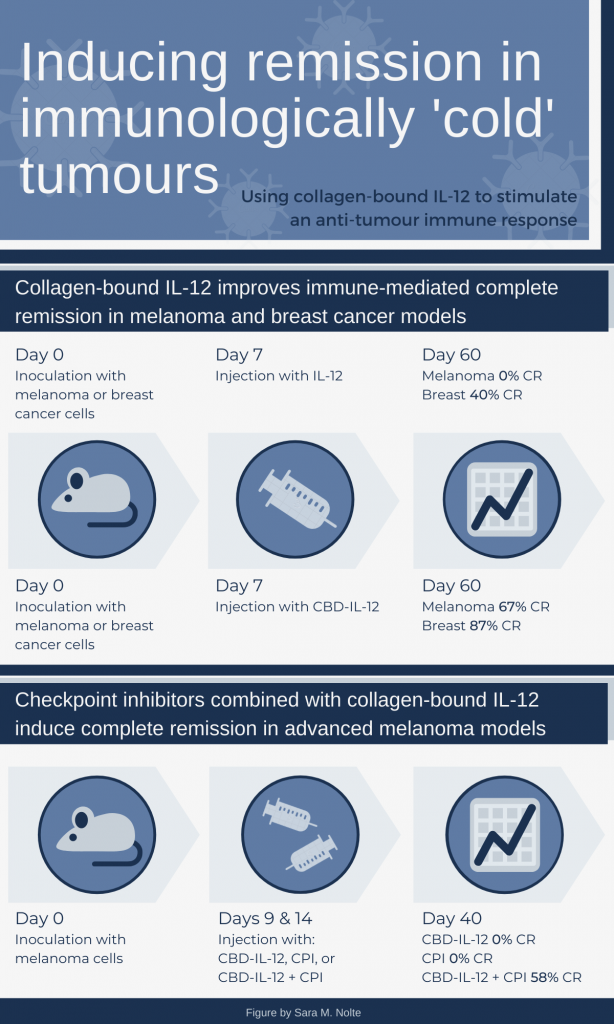
Using mouse models for melanoma and triple negative breast cancer, the researchers demonstrated that a single injection of CBD-IL-12 resulted in significantly higher complete remission (CR) rates, compared to IL-12 only treatment. In the advanced melanoma model, two doses of CBD-IL-12 led to significantly reduced tumour burden and prolonged survival, compared to CPI only treatment. However, CR was only achieved when mice were treated with CBD-IL-12 and CPIs in combination .
You might wonder about the discrepancies in the CBD-IL-12 CR rates between the two sets of experiments (67 versus 0 percent). Recall that the CPI experiments use a different methodology, resulting in a more extensive tumour burden at the time of initial treatment, which likely had a big role in the outcome of the single therapy. This approach reflects a not uncommon situation in human cancers. More extensive disease often requires a multi-pronged approach and/or longer duration of treatment.
In addition to improved anti-tumour immune responses, the authors were able to demonstrate that CBD-IL-12 reduced systemic immune effects. An unchecked IL-12 mediated immune response can run rampant and cause toxic effects to the host. However, mice treated with CBD-IL-12 had preferential accumulation of activated T cells and inflammatory molecules in the tumour, and reduced markers of organ damage.
The take-home points:
- Collagen-bound IL-12 successfully makes immunologically “cold” tumours hotbeds of immune activity, increasing immune-mediated remission;
- The effectiveness of CPIs is significantly improved with CBD-IL-12, making them promising therapies once again;
- This immune activity is localized to the tumours, protecting the host from immune-mediated toxicity.
And while those aspects of the study are promising for cancer immunotherapies, I was most excited by two smaller experiments:
- In the breast cancer model, CBD-IL-12 mice with CR were challenged with a second inoculation of tumour cells, but no second treatment injection. Growth of a second tumour was prevented in 90 per cent of the mice, suggesting retained immune memory.
- In a metastatic melanoma model, mice were treated with either IL-12 or CBD-IL-12. The percentage of lung metastases was reduced by 50 percent in the in CBD-IL-12 group, with an associated increase in active immune cells. This suggests the immune response can be triggered wherever the tumour cells end up.
I find these bits of data most exciting, as it suggests these immune responses could be helpful in preventing metastasis and/or recurrence of disease, which is a cause of significant mortality in melanoma and breast cancers.
The authors anticipate their next steps will focus on further exploring potential toxicological side effects of CBD-IL1-2 therapy, while improving its tumour-specificity and safety profile (see press release). Personally, I hope we get to see expansion of their immune memory findings and how they could potentiate prevention of relapse and/or metastasis.
Sara M. Nolte
Latest posts by Sara M. Nolte (see all)
- Ottawa researchers make tumour-marker negative cancers positive with oncolytic viruses - December 19, 2024
- Cell-based therapy approved as alternative to standard UCB transplant for hematological malignancies - June 21, 2023
- Finding the perfect match: breaking down the science of organ and stem cell transplant matching - April 18, 2023






Comments