The second post in this series on gene therapy and blood stem cells might give readers a small sense of the absolutely enormous research investment (time, money, people, etc.) required to find a way to expand blood stem cells for therapeutic purposes. Ever since the first bone marrow transplantations in the late 1950s, where the regenerative potency of blood stem cells was powerfully realized, researchers have been keen to develop methods to create unlimited pots of these cells for clinical use.
The scale of such a dream has not gone unnoticed in the bioengineering field, with numerous research and industrial programs dedicated to understanding how to scale-up blood stem cell production for use in cellular therapies with blood stem cells representing the initial or final product.
Despite these efforts, relatively little research time is directed toward understanding the most fundamental biophysical properties of blood stem cells. The effects of physical stresses (e.g., shear stress) and the role that structural elements (e.g., membrane stiffness, actin cytoskeleton) play on the maturation state of blood stem cells are largely unknown, and there is urgent need to tackle these underexplored research areas.
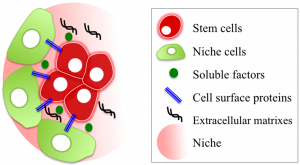
You might ask why researchers would care about physical and mechanical biology in a cell that makes blood – it’s all one big liquid after all, right? The thing is that blood stem cells live in a specialized area of the bone marrow (called the stem cell niche) that has been demonstrated to “hold onto” stem cells.
Indeed, a number of clinical agents have even taken advantage of this feature by releasing stem cells (a process called “stem cell mobilization”) from these niches to enter the blood stream. As an example, mobilized stem cells can then be harvested from the blood, thereby avoiding the need for a bone marrow sample, which is quite a painful procedure and a deterrent for adult stem cell donation).
Knowledge of this physical home with physical molecules anchoring the stem cells in place has not gone completely unnoticed, but over the last decades the majority of stem cell biologists have typically shied away from trying to mimic the physical home of blood stem cells in favour of trying to establish the molecules driving biological processes.
Whereas the latter is certainly important from a targeted therapy point of view, it has left the biophysical properties of blood stem cells as a complete unknown and this represents a substantial knowledge gap in informing efforts to scale-up stem cell therapies. The development of novel biocompatible artificial niches for stem cell transplantation could easily be a space where major breakthroughs are made in the next 3-5 years.
Experiments in this space do have a long history with the pioneering work of people like Mike Dexter and Ray Schofield in the late 1970s, demonstrating that the bone marrow cells that weren’t blood stem cells could act as a supportive niche for stem cell expansion. Numerous groups followed suit, developing hundreds of cell lines from dozens of tissues with different levels of support, but working out exactly which cell or which molecules were critical for stem cell maintenance and expansion has remained a complex problem that has not been solved by testing one molecule at a time.
For those interested in reading more, Mendez-Ferrer et al. and Beerman et al. have published excellent articles that summarize recent efforts to identify and label supportive cells in the bone marrow, but much debate remains and it is unlikely to be a single molecule on a single cell.
On the other end of things, researchers have also tried creating a synthetic niche for blood stem cells to grow in, using artificial biocompatible materials to try and modify stiffness, elasticity, molecular composition, etc. Perhaps the most exciting of these was a recent effort by Bourgine et al., which tried to mimic the bone marrow niche including a “bone-like scaffold, functionalized by human stromal and osteoblastic cells and by the extracellular matrix they deposited during perfusion culture in bioreactors.”
This approach represents one of the more “biologically inspired” artificial niches by engineering cells to deposit relevant niche molecules in different amounts, thereby retaining the “tunability” of completely artificial matrices. Still, progress toward defining the exact components of these sorts of more biologically relevant niches will be challenging and this will need to be coupled with rigorous functional assays to ensure that stem cell function is retained.
I would be remiss not to mention that a number of studies have focused their attention on understanding the range of different blood stem cell niches in development, at a time when blood stem cells must undergo massive expansion – as opposed to the “maintenance mode” they exist in throughout adulthood (when they are living in the bone marrow).
Still other studies have demonstrated the importance of regulating negative feedback of cells and secreted molecules, a job likely regulated by a dynamic in vivo bone marrow environment, but virtually impossible to study in situ. Again, for those interested in reading further, Kumar and Geiger have summarized research efforts well in their recent review.
Overall, bringing these areas together is essential to understand fully how blood stem cells live and expand normally. We must think of ways to explore the numerous potential regulators of blood stem cells in more complex artificial microenvironments, whilst also trying to strip things down to minimal components to study the individual mechanical and biophysical properties that bioengineers can learn and build from.
This will be a huge multi-disciplinary effort that really requires experts at the leading edge of different fields – biomaterials, blood stem cells, mathematical modeling, engineering, etc., – to come together to tackle. Exciting times ahead!
David Kent
Latest posts by David Kent (see all)
- Breaking down barriers between industry and academia to accelerate gene therapy - August 22, 2023
- Reversing aging: Not just a single system to consider… - August 25, 2021
- Regenerative medicine and COVID-19: The search for a silver lining - August 26, 2020






Comments