“Regenerative medicine news under the microscope” is a monthly feature highlighting big stories in stem cell research. I sample the latest and greatest findings in recent press and package them into a single post.
In this double issue, I cover a gene therapy for neuropathic pain resulting from spinal cord injuries, the latest brain rejuvenation paper, a newly discovered “exercise molecule,” and more!
Pick of the Month(s)
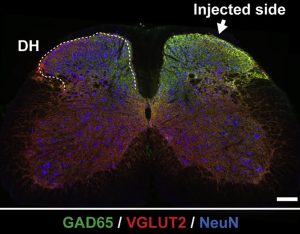
Cross section through the lumbar portion of mouse spinal cord. A green glow signals upregulation of inhibitory neuronal markers (GAD65) in target excitatory neurons (VGLUT), indicating a successfully engineered switch in molecular identity. This switch was key to the elimination of neuropathic pain in Tadokoro et al.2022, Molecular Therapy.
Promising gene therapy eliminates neuropathic pain caused by spinal cord injuries
It’s well known that spinal cord injuries can lead to sensory or motor deficits, and even paralysis. However, chronic pain may also result, including but not limited to neuropathic pain, which is generated by defects in parts of the nervous system responsible for reporting damage (click here to read more). Neuropathic pain can cause debilitating symptoms such as muscle weakness, numbness, tingling and pain. Unfortunately, there are no successful one-off treatments; drugs may be prescribed, but often delivery must be continuous to work and this can produce unwanted side effects such as motor weakness, sedation and, in the case of opioids, addiction.
Given that research on neuropathic pain has previously implicated excitatory neurons transmitting pain signals in the spinal cord, Tadokoro et al. used a genetic engineering strategy to convert excitatory neurons to inhibitory neurons in vivo. They targeted a specific area of the spinal cord – the dorsal horn – in a mouse model of sciatic nerve injury. Fantastically, this resulted in the elimination of neuropathic pain, an effect lasting 2.5 months after treatment. The authors also noted that no side effects were observed between two- and 13-months following treatment in an array of model organisms, including mice, pigs and primates. Look no further for an inspiring summer read.
One step closer to brain injury repair
Since the discovery of neural stem cells, scientists have been trying to determine how they can be applied to brain repair following events like traumatic injury or stroke. However, up until this past May, it was exceedingly difficult to maintain the developmental potential of certain progenitor pools, which is necessary for both bench and clinical applications. Enter Varga et al., who published a molecular cocktail designed to maintain cortical progenitor cells in a multipotent state.
Because these cells are fated to become cortical neurons, the authors refer to them as cortically-specified neuroepithelial stem cells (cNESCs). The cNESCs differentiate to become forebrain cells once the cocktail is withdrawn, forming lower- and upper-layer cortical tissue both in vitro and in vivo. Our neural stem cell readers are probably curious about the cocktail components, so here they are: Together with the activation of FGFR, they used inhibitors of BMPR, GSK3, TGFβR, AKT and TNKS, naming the concoction “6F.”
A critical next step is going to be investigating how well this strategy can result in functional recovery from injuries, such as movement and cognition. Check out the full University of Toronto news release here.
Youthful cerebrospinal fluid may rejuvenate elements of the aging brain
You’ve probably read Cal Strode’s recent piece on Signals discussing regenerative medicine and its potential to reverse aging. The current highlight fits right into that theme; however, cerebrospinal fluid (CSF) is of special interest here. CSF is a liquid that surrounds and perfuses central nervous system tissues to 1) act as a cushion to protect against injuries, and 2) provide critical nutrients to cells. This past May, Iram et al. showed that infusing an older mouse with the CSF from a young donor mouse may improve memory function, and that this phenomenon is produced by the signaling molecule Fgf17.
Specifically, they saw functional improvement in memory testing, plus increased proliferation and differentiation of oligodendrocyte precursors – cells critical for neuron signaling and metabolic support. These cells can be adversely affected by aging and age-related diseases, which is why this finding holds promise. For more on the topic, check out this paper published back in April.
Considering that bodily fluids like CSF are made up of hundreds of proteins, I always enjoy reading about the processes by which teams narrow down searches like these. This is a very interesting and thought-provoking read – though I’d recommend you also follow up with Dr. Paul Knoepfler’s take over at The Niche, since there might be an extra experiment or two you might have liked to see.
Harnessing the burn: Newly identified exercise molecule cycles back feeding and obesity
Though many of us want nothing more than a pill that contains all the benefits of a morning jog, science just isn’t there, yet. Still, this next story is incredibly interesting: Li et al. have identified a metabolite produced in the body during exercise that seems to suppress feeding in rodents, promoting a healthy body weight and keeping obesity at bay. This metabolite is N-lactoyl-phenylalanine, referred to in the paper as Lac-Phe, and it’s synthesized in CNDP2+ immune and epithelial cells across an array of organs. The metabolite results from the condensation of lactate (yes, that lactate, debunked as the source of post-workout muscle soreness in the ‘80s – read more here and here) and phenylalanine, catalyzed by the CNDP2 enzyme. If these findings track to humans, there are obvious clinical implications for this metabolite, but more investigation will definitely be required.
This technically isn’t the first exercise-linked molecule found to suppress appetite (see more on these previous findings here, here and here); however, those factors have additional metabolic roles, not all of which have been shown to overlap with Lac-Phe – at least, not yet. Also, based on their analysis, Lac-Phe seemed to be “the most significantly induced circulating metabolite” in both mice and racehorses, setting it apart from the rest.
For additional analyses of this finding, head over to Nature and read this piece by Kim & Sternson.
Pig heart used in xenotransplant was infected – potential cause of death?
We’ve been following David Bennet’s story closely. In the December 2021/January 2022 edition, I wrote about how he did not qualify for a human heart transplant, and instead received a genetically engineered pig heart. This past March, we learned that Mr. Bennet passed away in hospital, but nobody could be sure why. A deeply disappointing potential cause of death has now surfaced, one touted as “a well-known – and avoidable – risk” by Antonio Regalado for MIT Technology Review. A porcine megalovirus, an apparently preventable infection, was found in the heart. Revivicor, the biotech company the pig had been sourced from, declined to comment.
Though nobody is certain that the megalovirus was the sole cause of death, experts seem to be in agreement that this could have been a major contributing factor. Apparently, this virus is not able to infect human cells, but can catastrophically damage the pig organ.
I was shocked to find out that the doctors detected the virus in Mr. Bennet while he was still alive, and in fact took measures to try and treat him, but it was too late. Antonio Regalado covers the harrowing cascade of events in depth, so be sure to read his coverage.
Cell culture differentiation conditions optimized by AI
As scientists, we like to think we’ll be among the last to be replaced by artificial intelligence (AI). However, there are a few tasks we’d be happy to hand off, and that has already begun. Kanda et al. have created a robotic AI system with a batch Bayesian optimization (BBO) algorithm – a technique to optimize unknown functions that are expensive (data-wise) – for cell culture applications. In plain English, it’s a system in which one set of inputs is evaluated, and the results of this evaluation are then used to determine the next set of inputs to be tried, and so on. If you have an enormous set of data to work through though – say, 200 million configurations to test – you may want to work in parallel batches. This is the BBO algorithm in a nutshell, and it’s what Kanda et al. used to find the best culture conditions for the differentiation of retinal pigment epithelial (RPE) cells, which are critical for sight, from induced pluripotent stem cells. Taking just 185 days (rather than the two and a half years it should have taken humans alone), the BBO brought differentiation efficiency up from about 50 to 90 per cent by the end of the experiment. Of course, all of this couldn’t have been accomplished without the help of Maholo, the humanoid robot that physically carried out the experiments. If you want to know more, this press release has a thorough summary.
A note from Lyla:
It has been such a privilege to write this feature for Signals over the past year. However, as my graduation date approaches, I have made the decision to divert as much time as possible into my remaining thesis experiments. It is for this reason that I will be putting my blogging activities on hold, just until my PhD work is complete. Thank you all very much for reading “Regenerative Medicine News Under the Microscope!”
Additional recommendations
Here are some papers/headlines that I didn’t have room for above:
Living skin on a robot. Kawai et al. in Matter.
Mutant SRF and YAP synthetic modified mRNAs drive cardiomyocyte nuclear replication. Xiao et al. in The Journal of Cardiovascular Aging.
Subventricular zone adult mouse neural stem cells require insulin receptor for self-renewal. Chidambaram et al. in Stem Cell Reports.
Sweet success: world’s largest islet transplant program celebrates 20 years of changing lives for people with diabetes. Gillian Rutherford for The University of Alberta.
Intestinal stem cell aging signature reveals a reprogramming strategy to enhance regenerative potential. Nefzger et al. in npj Regenerative Medicine.
Thermo Fisher seeks dismissal of Henrietta Lacks’ family’s lawsuit regarding sale of her cells. Craig LeMoult for GBH.
Patient-derived and mouse endo-ectocervical organoid generation, genetic manipulation and applications to model infection. Gurumurthy et al. in Nature Protocols.
Mobilization-based chemotherapy-free engraftment of gene-edited human hematopoietic stem cells. Omer-Javed et al. in Cell.
Molecular profiling of stem cell-derived retinal pigment epithelial cell differentiation established for clinical translation. Petrus-Reurer et al. in Stem Cell Reports.
Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Qi et al. in Nature Medicine.
A modular 3D printed microfluidic system: a potential solution for continuous cell harvesting in large-scale bioprocessing. Ding et al. in Bioresources and Bioprocessing.
Adenine Base Editing In Vivo with a Single Adeno-Associated Virus Vector. Zhang et al. in GEN Biotechnology.
In situ 3D bioprinting with bioconcrete bioink. Xie et al. in npj Regenerative Medicine.
Is embryonic stem cell research a target after Roe? Paul Knoepfler at The Niche.
Glucocorticoid signaling and regulatory T cells cooperate to maintain the hair-follicle stem-cell niche. Liu et al. in Nature Immunology.
Single-cell transcriptomic profiling of lung endothelial cells identifies dynamic inflammatory and regenerative subpopulations. Zhang et al. in JCI Insight.
Proteoglycan 4 (PRG4) treatment enhances wound closure and tissue regeneration. Krawetz et al. in npj Regenerative Medicine.
Paolo Macchiarini Found Guilty for Botched Surgery. Amanda Heidt for The Scientist.
Human umbilical cord mesenchymal stem cell-derived treatment of severe pulmonary arterial hypertension. Hansmann et al. in Nature Cardiovascular Research.
Schwann cells in the subcutaneous adipose tissue have neurogenic potential and can be used for regenerative therapies. Stavely et al. in Science Translational Medicine.
First patient in the Netherlands successfully treated with stem cell gene therapy. Universiteit Leiden News.
Another topic double-feature – bone regeneration: Mussel-inspired multifunctional surface through promoting osteogenesis and inhibiting osteoclastogenesis to facilitate bone regeneration. Wu et al. in npj Regenerative Medicine. AND A murine model of large-scale bone regeneration reveals a selective requirement for Sonic Hedgehog. Serowoky et al. in npj Regenerative Medicine.
Publication of the ‘Google Maps’ of human cells is a milestone. A pioneer of the project explains why. Megan Molteni for STAT.
Intrinsic epigenetic control of angiogenesis in induced pluripotent stem cell-derived endothelium regulates vascular regeneration. Macklin et al. in npj Regenerative Medicine.
Patient-derived micro-organospheres enable clinical precision oncology. Ding et al. in Cell Stem Cell.
Target receptor identification and subsequent treatment of resected brain tumors with encapsulated and engineered allogeneic stem cells. Bhere et al. in Nature Communications.
Metabolic reprogramming of skeletal muscle by resident macrophages points to CSF1R inhibitors as muscular dystrophy therapeutics. Babaeijandaghi et al. in Science Translational Medicine.
A cellular hierarchy framework for understanding heterogeneity and predicting drug response in acute myeloid leukemia. Zeng et al. in Nature Medicine.






Myself Sudha. I am 39 years old. I came at Advancells for Anti-aging and Rejuvenation of my body. My experience was very good. They gave me mesenchymal stem cell and PRP treatment for my skin.
I am happy to say that the people of Advancells – all staffs and lab team are very generous and cooperative.
Amazing work.