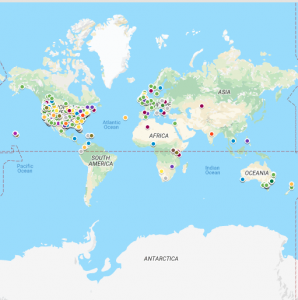
Recipient locations and destinations from one year of GoFundMe crowdfunding campaigns for stem cell treatments for neurological diseases and injuries. (Source: Drs. Snyder and Turner)
Jeremy Snyder, PhD, is a Professor in the Faculty of Health Sciences at Simon Fraser University. Leigh Turner, PhD, is an Associate Professor in the Center for Bioethics, School of Public Health and College of Pharmacy at the University of Minnesota. You can find them on Twitter @jeremycsnyder and @LeighGTurner
Direct-to-consumer (DTC) marketing of unproven and unlicensed stem cell-based interventions (SCBIs) has been rightly criticized on ethical, scientific and legal grounds. Businesses selling such purported treatments often attempt to evade regulatory restrictions designed to protect patient safety and public health. For example, in the U.S. and Canada, and elsewhere, many such businesses claim they do not require premarketing authorization from federal regulatory bodies, even though they promote cell-based products that are classified as drugs and biologics and require approval from the U.S. Food and Drug Administration or Health Canada.
Moreover, the safety and efficacy of such products typically have not been established in credible clinical trials. Nonetheless, businesses selling putative stem cell treatments on a DTC basis often make unsupported claims concerning both safety and efficacy. Despite such representations, the use of unproven SCBIs has led to deaths, serious injuries and reports of fraud. Advertising claims about the risk-free or minimal risk nature of such procedures mean patients and prospective clients often are unaware that serious injuries can occur.
Numerous steps are being taken to combat DTC sales of unproven SCBIs, including more robust enforcement of regulations, development of more comprehensive and rigorous regulatory standards, and efforts to inform the public of risks associated with unproven SCBIs. One barrier to developing evidence-based patient safety and public education initiatives is that our knowledge of the DTC marketplace is incomplete. While some research has been published on DTC businesses in the U.S., Canada and other countries, and on the specialties of physicians working at such facilities, much less is known about individuals seeking to purchase SCBIs. This knowledge gap includes the countries of origin of patients seeking unproven stem cell interventions, the DCT businesses to which they travel, and the diseases they hope to treat.
To obtain answers to some of these important questions, we analyzed medical crowdfunding campaigns from the crowdfunding platform GoFundMe. We focused on campaigns for neurological diseases and injuries because reviews of DTC business websites show this is a very common category for these businesses and they raise significant health concerns for patients. While only some patients accessing unproven SCBIs via DTC businesses will crowdfund to pay for such procedures, we hoped that analyzing such campaigns would provide a general picture of the practice as few public and private insurers pay for these interventions and many individuals face significant financial difficulties in purchasing them.
We identified 1,030 relevant crowdfunding campaigns initiated between November 2017 and 2018. The most common neurological conditions for which donations for stem cell procedures were sought included multiple sclerosis (MS) at 35.5% of campaigns, followed by autism (9.5%), spinal cord injury and paralysis (8.9%), cerebral palsy (7.1%), and stroke (5.0%). In part due to GoFundMe’s dominance in North America and English-speaking countries, these campaign recipients were generally from the U.S. (78.1%), followed by the U.K. (7.2%), Canada (5.3%) and Australia (3.1%).
While this is a global marketplace in which some individuals travel great distances seeking purported stem cell therapies, different clinics and neurological conditions exhibit distinct patterns of interest among patients. For example, Panama’s Stem Cell Institute and Mexico’s Clinica Ruiz drew globally for patients whereas the U.S.-based businesses drew much more from the U.S. and Canada. These travel patterns can be explored using our map of businesses and campaigner locations.
We were surprised by some of our study findings. For example, we had not anticipated the dominance of MS campaigns as there are many times more people with Alzheimer’s disease, stroke, epilepsy and brain injuries in the U.S. The outsized proportion of MS campaigns may be due to high profile stories of promising research on hematopoietic stem cell transplantation treatment for MS and the number of businesses that market supposed stem cell treatments for MS. In our study, these included DTC businesses catering to MS patients like Mexico’s Clinica Ruiz and Russia’s AA Maximov Hospital.
We were also struck by the number of campaigns that were related to SCBIs administered at academic centres, namely Northwestern and Duke Universities. While we do not lump academic institutions conducting clinical trials with clinics using misleading advertising claims to sell unlicensed and unproven SCBIs, we wish to draw attention to crowdfunding activity prompted by the substantial expenses associated with pursuing stem cell transplants at these academic institutions. “Stem cell hype” generated by universities could contribute to such crowdfunding activity.
The most active DTC businesses in this area included Panama’s Stem Cell Institute, which was previously forced out of Costa Rica due to regulatory changes, and California’s StemGenex, which has received a warning letter from the FDA for failing to comply with federal regulations. StemGenex is also the subject of a class action lawsuit brought by former patients who allege they purchased SCBIs on the basis of fraudulent marketing claims. Having this information can help investigators and regulators who wish to focus on DTC businesses with the largest clientele and greatest impact.
We believe that having this information is important for stem cell researchers, policy-makers, regulatory bodies, patients and other parties concerned about the risks DTC marketing of SCBIs pose to patient safety and public health. Having an empirically informed understanding of the size and geographic distribution of the clientele of this marketplace is essential for those wishing to ensure that policy responses are coordinated, evidence-based, effective and tailored to specific audiences.
Guest
Latest posts by Guest (see all)
- Regenerative immunotherapy: Hope for chronic autoimmune diseases - September 16, 2025
- Canada’s regenerative revolution: Why AI is the catalyst - September 4, 2025
- Summer by Design: A launchpad for future entrepreneurs and industry scientists - August 14, 2025






Comments