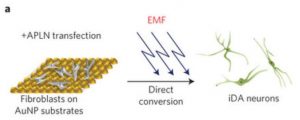
Electromagnetized gold nanoparticles mediate direct lineage reprogramming into induced dopamine neurons in vivo for Parkinson’s disease therapy (used with permission Springer Nature, Nature Nanotechnology)
The ability to revert a terminally differentiated, somatic cell back to a pluripotent stem cell state has been of incalculable value to researchers since its discovery in 2006. In recent years, researchers have modified this technique by cutting out a step, allowing somatic cells to be directly reprogrammed into a new somatic cell type without first having to become a stem cell intermediary. Direct cell reprogramming is regarded as a simple method to obtain large numbers of specific cell types that can otherwise be tricky or impossible to harvest from the body.
Current methods of direct cell reprogramming include the use of chemical cocktails that induce the cell to change its identity by forced expression of key transcription factors in the genome. This method is effective, but not without limitations. Chemicals can be tricky to use; cells must be exposed to a uniform concentration over time, they need to be replenished in the culture medium often, and there can be chemical batch-to-batch variability that can affect the efficiency of cellular reprogramming. That is why researchers are now looking at alternative methods to achieve the same reprogramming results.
Electromagnetic field (EMF) reprogramming is a nascent alternative method to the use of chemicals. EMFs are physical energy fields generated by electrically charged objects, many of which humans are exposed to daily from electronic devices, as well as the Earth’s natural magnetic field. To reprogram cells, an EMF is generated and applied to electrically charged objects, to which cells can then be exposed. Electrical fields have been shown to affect a surprising number of cellular processes via epigenetic changes; that is, they affect which genes are turned on and off in a cell, much in the way small molecule chemicals would, but through a different mechanism.
A study from Yoo et al, published in Nature Nanotechnology, demonstrates the effectiveness of EMF lineage reprogramming to generate dopaminergic neurons for Parkinson’s therapy. Electromagnetic energy was transferred to cultured mouse and human fibroblasts with the use of gold nanoparticles (AuNPs), which are exposed to EMF frequencies to become magnetized before being coated on glass slides.
The researchers demonstrated that when fibroblasts are exposed to magnetized AuNPs, they upregulate neuron-specific markers, and remarkably their genome was found to more closely resemble primary dopaminergic neurons than neurons obtained by chemical reprogramming. When probed further, the researchers determined that the histones – proteins in the cell nucleus that tightly wrap DNA – are acetylated after EMF exposure, meaning that the DNA they contain becomes unravelled in certain regions called promoters. The specific promoters exposed by this method drive the transcription of genes that encode for neurons, causing the fibroblasts to switch phenotypes and become dopaminergic neurons.
Having demonstrated the mechanism of reprogramming in cell culture, the researchers next tested their technology to reprogram endogenous cells of the brain in an animal model. Previous attempts at reprogramming resident cells in an organism have been largely unsuccessful and involve the complex delivery of chemicals or gene therapy.
They induced Parkinson’s symptoms in two different mouse models and injected AuNPs into the striatum of the brain. Animals were then exposed to an EMF for three hours per day for four weeks, and changes in motor function and brain tissue were investigated.
The researchers reported that animals treated with AuNPs + EMF had significant motor recovery and more dopaminergic neurons present in the brains. Lineage tracing of the reprogrammed neurons revealed that they were derived from the support cells of the brain – astrocytes – that responded to the EMF treatment and underwent reprogramming. A minor drawback to the results was that the researchers unfortunately did not include an experimental group exposed to EMFs without AuNPs, so it is unknown if EMFs alone may have affected the study outcome.
While this technology could be readily applied for reprogramming cells in a dish, the remarkable implications for future applications (for example, to change undesirable disease-state cells in the body into a more desirable or therapeutic cell type without the use of drugs or chemicals) are not without limitations and concerns. The AuNPs, although safe and biocompatible, must be delivered into a specific area of the body, necessitating invasive surgery or the use of an as-yet-to-be-developed targeting mechanism.
Furthermore, the translation from animal model to human use comes with many unknowns: what distance can EMFs penetrate tissue to reach the AuNPs? Logistically, how practical is receiving daily EMF exposure for patients? Lastly, does exposure of EMFs have any off-target effects that may be of concern? Future research will hopefully aim at addressing some of these questions to improve the feasibility of this technology for regenerative medicine therapies.
Samantha Payne
Latest posts by Samantha Payne (see all)
- Growing pains for the regenerative medicine industry - August 29, 2018
- It’s electrifying! Cell reprogramming using electromagnetic fields - May 24, 2018
- Adapting the language of computers for regenerative medicine - February 16, 2018






Comments